Field potential Imaging in human iPSC- derived Cardiomyocytes using UHD-CMOS-MEA
Field potential Imaging in human iPSC- derived Cardiomyocytes using UHD-CMOS-MEA
Matsuda, N.; Nagafuku, N.; Matsuda, K.; Ishibashi, Y.; Taniguchi, T.; Matsushita, Y.; Miyamoto, N.; Yoshinaga, T.; Suzuki, I.
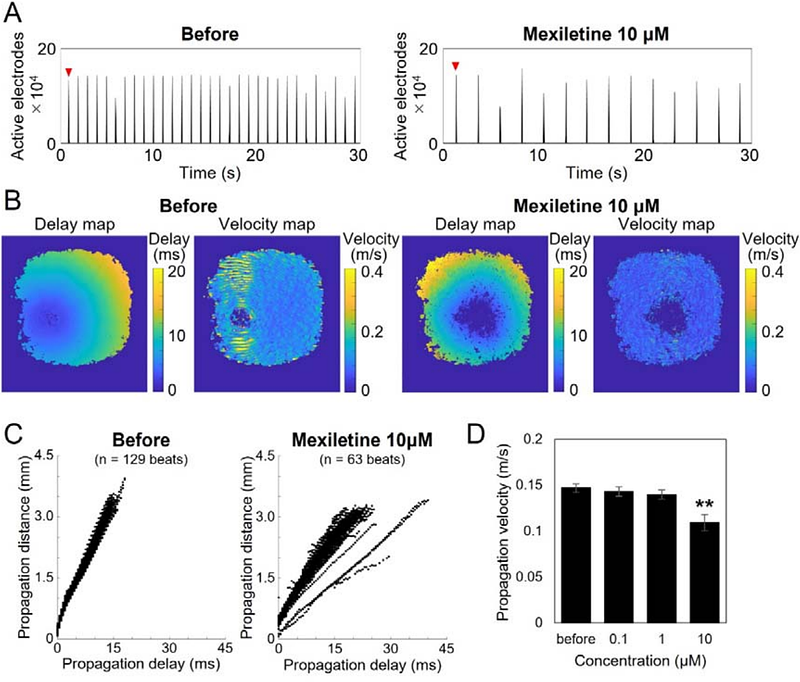
AbstractEvaluation using beat propagation analysis of human iPS cardiomyocytes is an effective approach for assessing human cardiac safety in drug development. However, current applications are primarily focused on detecting QT prolongation and arrhythmia risk, while its ability to comprehensively detect cardiotoxicity remains insufficient. Additionally, predicting the mechanism of action, which is crucial in drug development, remains challenging. In this study, we employed field potential imaging (FPI) using an ultra-high-density (UHD) CMOS microelectrode array (MEA) comprising 236,880 electrodes with high spatiotemporal resolution, capable of recording the activity of a monolayer of cardiomyocytes with tens of electrodes per cell. This method enabled the establishment of novel electrophysiological endpoints, including the number of excitation origins, fluctuations in origin positions, conduction velocity, and propagation area. Pharmacological characterization revealed drug-specific effects: isoproterenol increased excitation origins, mexiletine reduced conduction velocity, and E-4031 decreased propagation area while inducing early afterdepolarizations. Multivariate analysis of 13 compounds across 17 electrophysiological endpoints distinguished conduction velocity and propagation patterns based on their mechanisms of action. Additionally, 0.1 M doxorubicin exposure for 24 hours significantly reduced conduction velocity and propagation area, allowing early detection of chronic cardiotoxicity. These findings suggest that UHD-CMOS-MEA-based FPI enhances cardiotoxicity detection at low concentrations and precisely characterizes ion channel activity across different drug concentrations. The integration of novel electrophysiological endpoints derived from UHD-CMOS-MEA-based FPI, including excitation origin analysis, conduction velocity, and propagation area, along with multivariate analysis, is anticipated to establish a next-generation in vitro platform for comprehensive cardiotoxicity risk assessment and mechanism-based prediction of drug candidates.