METTL5 Blockade Enhances Anti-Tumor Immune Response via Inducing Neoantigen Generation
METTL5 Blockade Enhances Anti-Tumor Immune Response via Inducing Neoantigen Generation
Zhang, Y.; Shi, X.; Wang, Y.; Wang, R.; Cao, Y.; Chen, H.
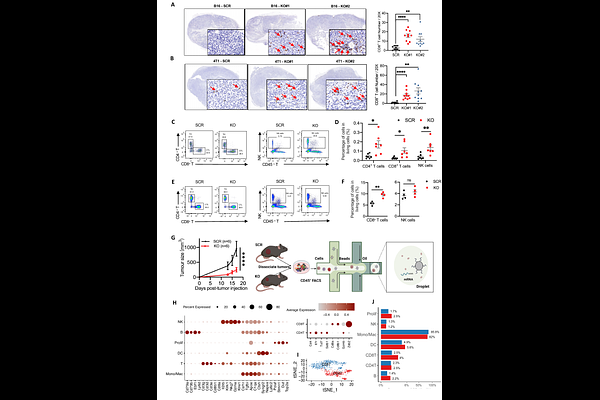
AbstractTumor neoantigens play a pivotal role in immunotherapy through eliciting tumor-specific immune responses. However, their clinical application is limited by the restricted repertoire of naturally occurring neoantigens, which undergoes continuous immune editing within the tumor microenvironment. To address this critical challenge, we explored translational regulation as a reversible and safer strategy to augment neoantigen production. Through systematic analysis of translational regulatory factors associated with immunotherapy response, we identified METTL5 as a key modulator of anti-tumor immunity. METTL5 depletion in syngeneic tumor models demonstrated significant enhancement of endogenous anti-tumor immunity across distinct MHC-I haplotypes, with synergistic effects observed when combined with immune checkpoint blockade. Based on evolutionary conservation of ribosomal decoding center modifications and established mechanisms from prokaryotic studies, we propose that METTL5-mediated m6A modification at the small ribosomal subunit decoding center may contribute to translational fidelity regulation. Mechanistically, integrated MHC-I immunoprecipitation-mass spectrometry and ribosome profiling revealed the identification of non-canonical peptides, including those derived from novel unannotated open reading frames (nuORFs), which provide previously absent neoantigen sources for tumor cells. T-cell receptor sequencing further demonstrated significantly enhanced clonal diversity and tumor-specific reactivity of infiltrating T cells in METTL5-deficient tumors. Therapeutic vaccination strategies targeting METTL5-deficiency-induced neoantigens, including both peptide-based and mRNA vaccines, exhibited partial anti-tumor efficacy in murine tumor models. This study establishes METTL5 as a critical translational regulator that restricts neoantigen diversity and proposes a novel combinatorial strategy to enhance immunotherapy through translational control.