DNA Polymerase α has pyrimidine dimer translesion activity that is suppressed during normal replication
DNA Polymerase α has pyrimidine dimer translesion activity that is suppressed during normal replication
Mukherjee, P.; Sarker, A.; Schauer, G.
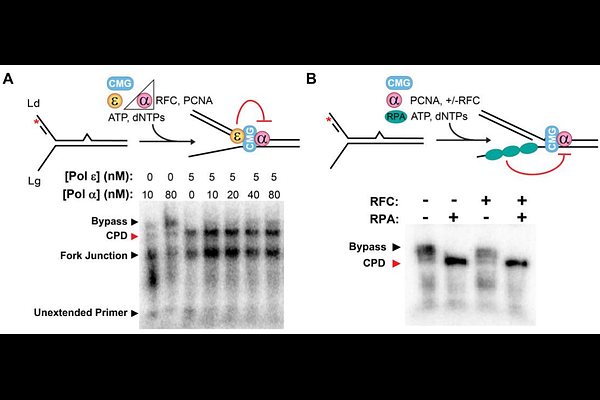
AbstractDNA is damaged by UV light, forming lesions like pyrimidine dimers which impede DNA replication forks and can cause mutations that lead to cell death or cancer. To ensure rapid and accurate DNA replication during S-phase, the cell uses so-called DNA damage tolerance (DDT) pathways to bypass this damage, leaving lesions to be repaired after completion of replication. One established DDT pathway is Translesion Synthesis (TLS), in which lesions are bypassed by specialized TLS Polymerases that work in conjunction with the replisome. Here, we demonstrate that DNA Polymerase alpha (Pol ), the primase/polymerase holoenzyme that primes daughter templates during DNA replication, can also unexpectedly function as a TLS polymerase in vitro. We use biochemical and single-molecule fluorescence assays as well as yeast genetics to characterize the cyclobutane pyrimidine dimer (CPD) TLS activity of Pol . The results indicate that Pol can robustly bypass CPD lesions with its native polymerase activity and without misincorporation of ribonucleotides. We demonstrate with single-molecule Fluorescence Resonance Energy Transfer (FRET) that the DNA binding cleft of Pol may remain open to accommodate a bulky CPD lesion during TLS. However, we also show that overexpression of Pol in vivo cannot rescue UV sensitivity of a TLS knockout strain lacking all three natively expressed TLS Pols. We further observe that the single-stranded binding protein, RPA, as well as Pols {epsilon} and {delta} each inhibit Pol CPD TLS activity. Conversely, these replisome components do not inhibit and can even stimulate Pol , the canonical TLS Pol for CPD lesions. The results suggest that, during normal replication, the intrinsic TLS activity of Pol is likely suppressed at the replication fork by the replisome itself.