Advanced Cardiac Organoid Model for Studying Doxorubicin-Induced Cardiotoxicity
Advanced Cardiac Organoid Model for Studying Doxorubicin-Induced Cardiotoxicity
Wu, X.; Williams, S.; Robidoux, J.; Sriramula, S.; Abdel, A.-R.
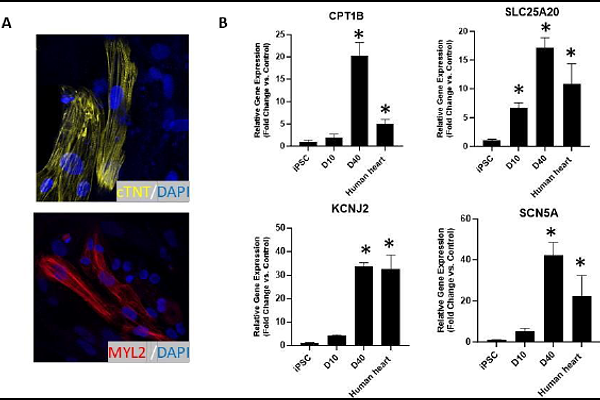
AbstractCardiac organoids provide an in vitro platform for studying heart disease mechanisms and drug responses. However, a major limitation is the immaturity of cardiomyocytes, restricting their ability to mimic adult cardiac physiology. Additionally, the inadequacy of commonly used extracellular matrices (ECM), which fail to replicate the biochemical and mechanical properties of natural heart tissue, poses significant challenges. Consequently, structural integrity in cardiac organoids is impaired. Moreover, scalability remains an obstacle, as conventional ECM substitutes hinder mass production of organoids for high-throughput toxicology screening. To overcome these challenges, we developed an advanced model promoting fibroblast-driven ECM self-secretion, enabling physiologically relevant tissue architecture and function. Using the ECM-free, mature cardiomyocyte-integrated organoid model, we investigated the cardiotoxicity of doxorubicin, a widely used chemotherapeutic agent known to impair cardiac function. Cardiomyocytes derived from induced pluripotent stem cells were characterized for maturity by immunostaining for cTNT and MYL2 alongside gene expression analysis. Organoids treated with doxorubicin showed reduced size and increased collagen deposition. These structural changes correlated with functional impairments, including decreased contraction rate and disrupted synchronous beating. In 2D culture, exposure to doxorubicin induced fibroblast activation, promoted endothelial-to-mesenchymal transition in endothelial cells, and triggered cytotoxic effects in cardiomyocytes. This study highlights the importance of ECM remodeling in advancing cardiac organoid models and demonstrates its potential for more accurate cardiotoxicity assessment. Addressing these limitations enhances the physiological relevance of cardiac organoid systems for drug safety assessment and cardiac disease modeling.