High burden of premature ventricular contractions upregulates transcriptional markers of inflammation and promotes adverse cardiac remodeling linked to cardiomyopathy
High burden of premature ventricular contractions upregulates transcriptional markers of inflammation and promotes adverse cardiac remodeling linked to cardiomyopathy
Medina-Contreras, J. L.; Balderas-Villalobos, J.; Gomez-Arroyo, J.; Hayles, J.; Kaszala, K.; Tan, A. Y.; Samso, M.; Huizar, J. F.; Eltit, J. M.
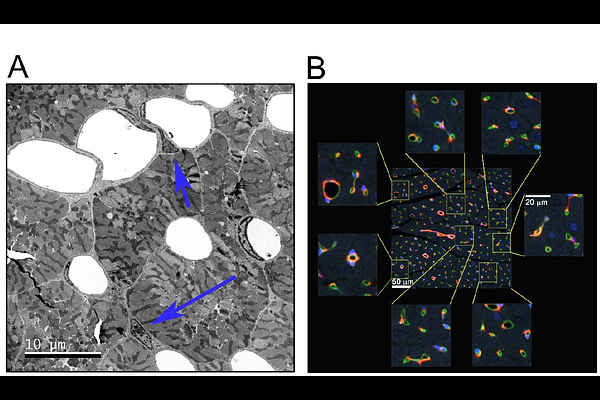
AbstractPremature ventricular contractions (PVCs) are the most prevalent ventricular arrhythmia in adults. High PVC burden can lead to left ventricular (LV) systolic dysfunction, eccentric hypertrophy, and an increased risk of heart failure (HF) and sudden cardiac death (SCD). Inadequate angiogenesis is a key determinant in the transition from adaptive to maladaptive cardiac hypertrophy and fibrosis is a risk factor for arrhythmia and SCD. To quantitatively assess structural remodeling and transcriptional alterations in PVC-induced cardiomyopathy (PVC-CM), animals were implanted with modified pacemakers to deliver bigeminal PVCs (200-220 ms coupling interval) for 12 weeks. Collagen deposition and interstitial ultrastructure of LV samples were analyzed using light and transmission electron microscopy, respectively. Pericytes, fibroblasts, myocytes, smooth muscle, and endothelial cells were imaged using confocal microscopy, quantified with an artificial intelligence-based segmentation analysis, and compared using hierarchical statistics. Transcriptional changes were assessed via RNAseq. Although cardiomyocytes hypertrophied in PVC-CM, capillary rarefaction was overcome by an increase in capillary-to-myocyte ratio. Additionally, thicker blood vessels were more abundant in PVC-CM. Fibroblast-to-myocyte ratio more than doubled, interstitial collagen fibers increased, and interstitial space thickened in PVC-CM. Transcripts involved in interstitial remodeling, inflammatory response, and alarmins were strongly elevated in PVC-CM. Overall, while the angiogenic response meets the metabolic demands of cardiac hypertrophy, upregulated markers of inflammation and cardiomyopathy linked to reactive fibrosis collectively represent an adverse LV remodeling that heightens the risk of HF and SCD in PVC-CM.