Ataxia-telangiectasia mutated (Atm) disruption sensitizes spatially-directed H3.3K27M/TP53 diffuse midline gliomas to radiation therapy
Ataxia-telangiectasia mutated (Atm) disruption sensitizes spatially-directed H3.3K27M/TP53 diffuse midline gliomas to radiation therapy
Mangoli, A.; Wu, S.; Liu, H. Q.; Aksu, M.; Jain, V. J.; Foreman, B. E.; Regal, J. A.; Weidenhammer, L. B.; Stewart, C. E.; Guerra Garcia, M. E.; Hocke, E.; Abramson, K.; Williams, N. T.; Luo, L.; Deland, K.; Attardi, L.; Abe, K.; Hashizume, R.; Ashley, D. M.; Becher, O. J.; Kirsch, D. G.; Gregory, S. G.; Reitman, Z. J.
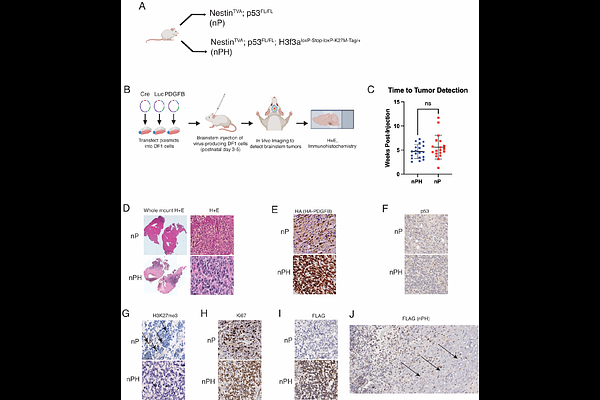
AbstractDiffuse midline gliomas (DMGs) are lethal brain tumors characterized by p53-inactivating mutations and oncohistone H3.3K27M mutations that rewire the cellular response to genotoxic stress, which presents therapeutic opportunities. We used RCAS/tv-a retroviruses and Cre recombinase to inactivate p53 and induce K27M in the native H3f3a allele in a lineage- and spatially-directed manner, yielding primary mouse DMGs. Genetic or pharmacologic disruption of the DNA damage response kinase Ataxia-telangiectasia mutated (ATM) enhanced the efficacy of focal brain irradiation, extending mouse survival. This finding suggests that targeting ATM will enhance the efficacy of radiation therapy for p53-mutant DMG but not p53-wildtype DMG. We used spatial in situ transcriptomics and an allelic series of primary murine DMG models with different p53 mutations to identify transactivation-independent p53 activity as a key mediator of such radiosensitivity. These studies deeply profile a genetically faithful and versatile model of a lethal brain tumor to identify resistance mechanisms for a therapeutic strategy currently in clinical trials.