Enhancing the protein fitness of interferon-lambda through computational design and glyco-engineering for prophylactic nasal drugs against respiratory viruses
Enhancing the protein fitness of interferon-lambda through computational design and glyco-engineering for prophylactic nasal drugs against respiratory viruses
Yun, J.; Yang, S.; Kwon, J. H.; Vecchietti, L. F.; Cha, M.; Chung, H. J.; Oh, J. E.; Kim, H. M.
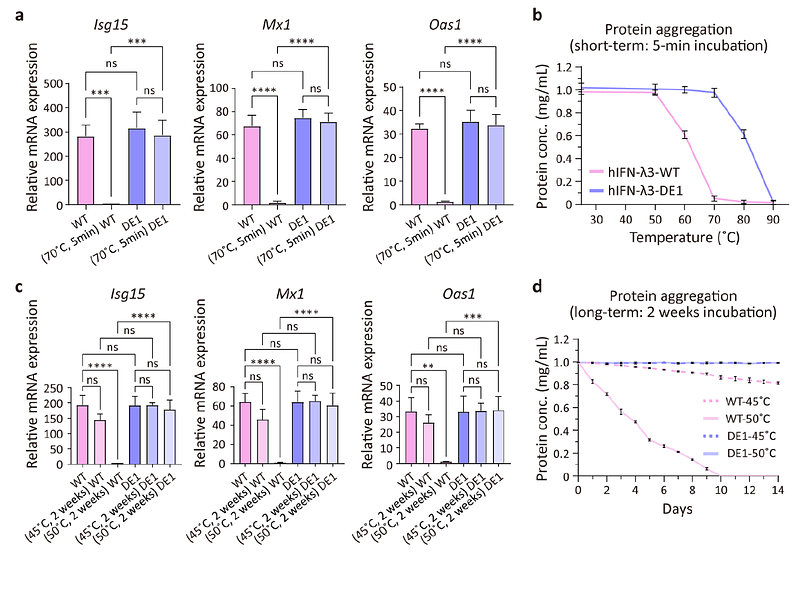
AbstractInterferon-{lambda}, a type III interferon that selectively targets epithelial cells, holds strong potential as an intranasal antiviral due to its ability to suppress respiratory virus replication without inducing systemic inflammation. However, clinical translation of human IFN-{lambda} is hindered by limited thermostability, protease susceptibility, and rapid mucosal clearance. In this study, instability-prone elements in hIFN-{lambda} are eliminated through artificial intelligence (AI)-based backbone remodeling and targeted surface hydrophobic patch engineering. A protease-sensitive loop is replaced with a de novo -helix, which shields neighboring hydrophobic patches and forms a new hydrophobic core, yielding an engineered variant (hIFN-{lambda}-DE1) with enhanced thermostability (Tm >90{degrees}C), protease resistance, and preserved antiviral activity and structural integrity even after extended heat stress (two weeks at 50{degrees}C). Further glyco-engineering introduces an N-linked glycan at a site distant from receptor-binding interfaces, improving solubility, production yield, and diffusion through synthetic nasal mucus. Intranasal administration of the resulting variant (G-hIFN-{lambda}-DE1) enables effective mucosal penetration and provides robust prophylactic protection against influenza A virus infection in an in vivo mouse model. These findings highlight a robust and versatile strategy that combines AI-driven structural design with glyco-engineering to develop scalable, bioavailable, and functionally enhanced nasal biologics for respiratory virus prophylaxis.