Characterizing putative glycosyltransferases within the flagella glycosylation island (FGI) of Aeromonas hydrophila ATCC 7966T
Characterizing putative glycosyltransferases within the flagella glycosylation island (FGI) of Aeromonas hydrophila ATCC 7966T
Fulton, K. M.; Mendoza-Barbera, E.; Tomas, J. M.; Twine, S. M.; Smith, J. C.; Merino, S.
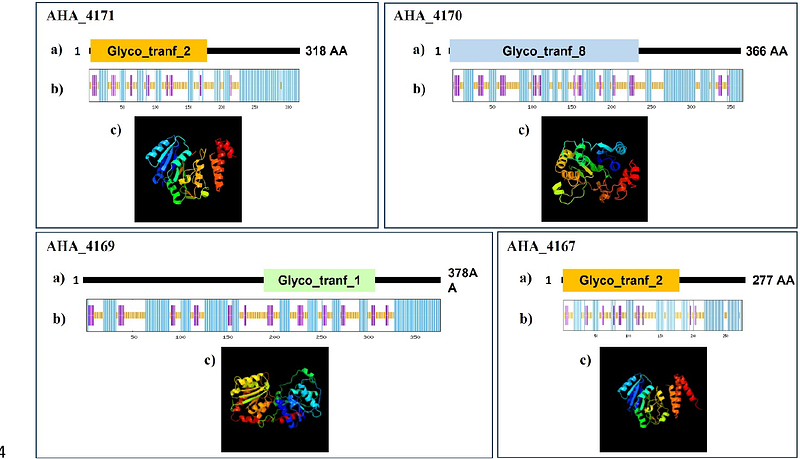
AbstractMotility is an important virulence factor for many pathogenic bacteria, enabling locomotion towards favourable conditions and away from hostile environments. Flagellar-mediated motility is driven by one or more flagellar filaments that extend outside of the cell and rapidly rotate to generate movement. These filaments are assembled through the polymerization of thousands of copies of structural flagellin proteins. It has been shown that flagellin glycosylation is often a prerequisite for proper flagella structure and function. Aeromonas hydrophila ATCC 7966T, a clinical and environmental pathogen, elaborates a single polar flagellum. The polar flagellin structural proteins FlaA and FlaB are glycosylated with a heterologous collection of complex penta- and hexa-saccharide chains. This study characterized the involvement of four genes with homology to known glycosyltransferases located within the A. hydrophila ATCC 7966T flagellar glycosylation island (FGI) in the biosynthesis of the complex polysaccharide glycans modifying the polar flagellins. Deletion of genes AHA_4167, AHA_4169, AHA_4170, and AHA_4171 were observed to have truncated glycans with sequentially shorter chain length, and all of these mutant strains had reduced motility compared to wild type bacteria.


