When less is not more: Limits to the evolution of metabolic dependence in spatially structured microbial communities
When less is not more: Limits to the evolution of metabolic dependence in spatially structured microbial communities
Ramesh, D.; Fara, E.; Oschmann, F.; Ugolini, G. S.; Stocker, R.; van Vliet, S.; Ackermann, M.; Schubert, O. T.
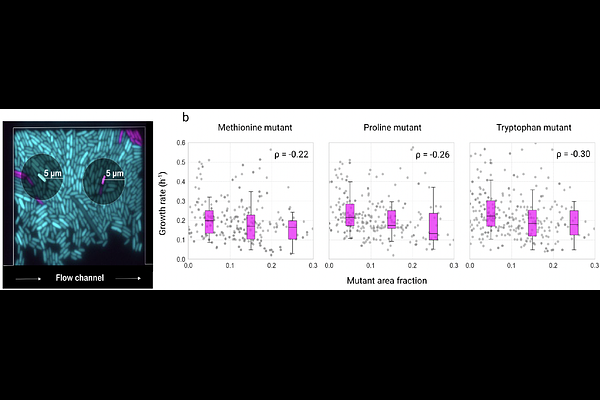
AbstractMost microbes grow in spatially structured, genetically diverse communities where they interact metabolically with neighboring cells. In such settings, evolutionary processes can result in the loss of biosynthetic pathways, leading to dependencies among strains. The Black Queen Hypothesis suggests that these gene losses reduce metabolic burden, potentially conferring a fitness advantage. However, how these dependencies evolve at the level of individual cells remains poorly understood. Here, we introduce an experimental system that enables direct observation of the first steps in the evolution of metabolic dependencies at single-cell resolution. Using a microfluidic setup combined with live-cell microscopy, we tracked the fate of individual amino acid auxotrophic mutant cells - deficient in methionine, tryptophan, or proline biosynthesis - within spatially structured Escherichia coli wildtype populations. When amino acids were externally supplied, auxotrophs grew faster than wildtype cells, Without external supply of amino acids, they grew significantly slower. When auxotrophs formed cell clusters, local depletion of amino acids further diminished their growth rates as well as the growth of neighbouring wildtype cells. Our mathematical model indicates that auxotroph growth is strongly constrained by the low rate of amino acid leakage from wildtype neighbors, conferring an advantage only once leakage surpasses a critical threshold. Overall, our findings show that the benefits of gene loss are highly context-dependent. Through single-cell experiments and modeling, we shed light on the early evolution of metabolic dependency and the trade-offs between reducing metabolic costs and maintaining metabolic autonomy in microbial communities.