Single particle dynamics of protein aggregation and disaggregation in the presence of the sHsp proteins IbpAB
Single particle dynamics of protein aggregation and disaggregation in the presence of the sHsp proteins IbpAB
Roth, A.; Puchalla, J.; Rye, H.
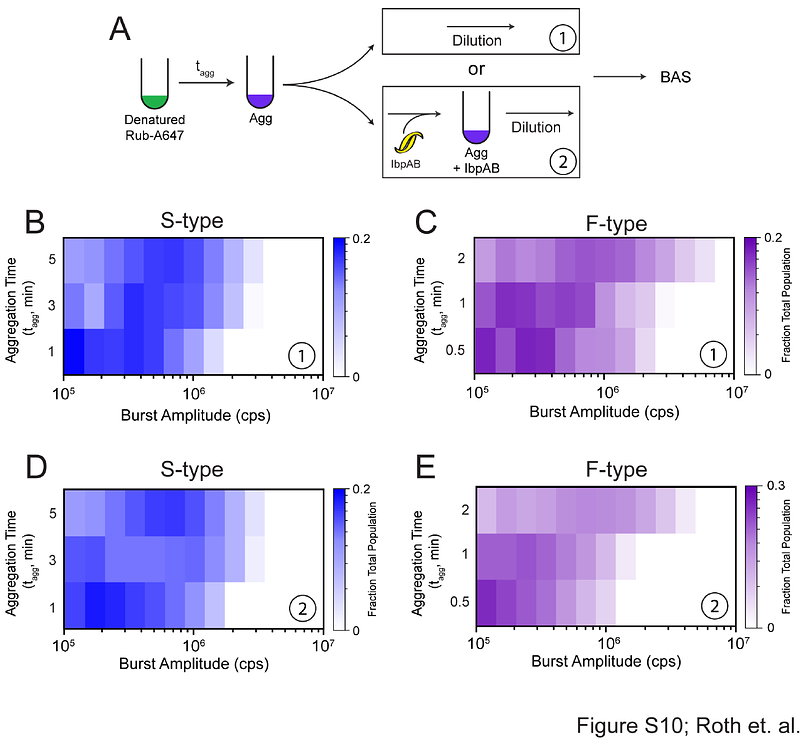
AbstractThe small heat shock proteins (sHsps) are a key class of molecular chaperones that can inhibit protein aggregation and enhance protein recovery from aggregates. However, the mechanisms sHsps employ to carry out these roles are not well understood, in part because the highly heterogeneous and dynamic particles they form with aggregating proteins are difficult to study with traditional biophysical tools. Here we have applied a novel single particle fluorescence technique known as Burst Analysis Spectroscopy (BAS) to the study of the E. coli sHsps IbpA and IbpB (IbpAB). We show that in the presence of IbpAB, two different model proteins converge toward similar, limited aggregate particle size distributions. Additionally, while IbpAB dramatically accelerates the disassembly of protein aggregates by the bacterial KJEB bi-chaperone disaggregase, this enhancement does not appear to be strongly influenced by aggregate particle size. Rather, it is the ability of IbpAB to alter aggregate structure during particle formation that appears to be essential for stimulated disassembly. These observations support a model of aggregate recognition by IbpAB that is not only highly adaptable but capable of shaping aggregate particles into a specialized range of physical properties that are necessary for efficient protein disaggregation.