GEMsembler: cross-tool structural comparison and ensemble modeling improve metabolic model performance
GEMsembler: cross-tool structural comparison and ensemble modeling improve metabolic model performance
Matveishina, E. K.; Bartmanski, B. J.; Benito-Vaquerizo, S.; Zimmermann-Kogadeeva, M.
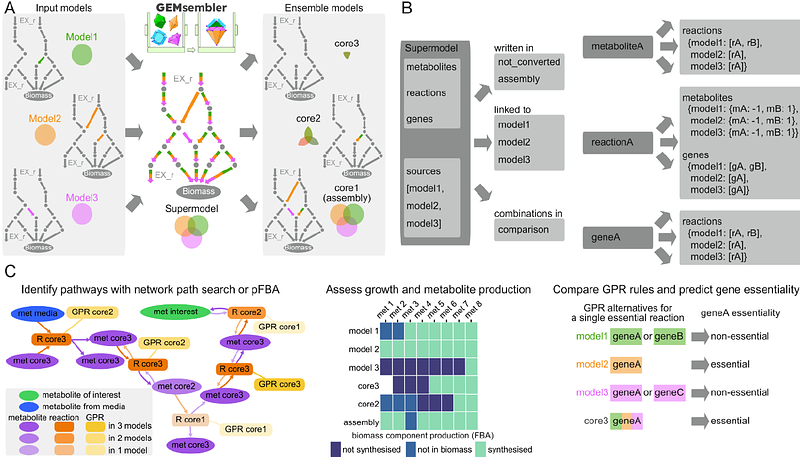
AbstractGenome-scale metabolic models (GEMs) are widely used in systems biology to investigate metabolism and predict perturbation responses. Automatic GEM reconstruction tools generate GEMs with different properties and predictive capacities for the same organism. Since different models can excel at different tasks, combining them can increase metabolic network certainty and enhance model performance. Here, we introduce GEMsembler, a Python package designed to compare cross-tool GEMs, track the origin of model features, and build ensemble models containing any subset of the input models. GEMsembler provides comprehensive analysis functionality, including identification and visualisation of biosynthesis pathways, growth assessment and agreement-based curation workflow. GEMsembler-curated ensemble models built from four Lactobacillus plantarum and Escherichia coli automatically reconstructed models outperform the gold-standard models in auxotrophy and gene essentiality predictions. Optimising gene-protein-reaction (GPR) combinations from ensemble models further improves gene essentiality predictions, even in the manually curated gold-standard models. GEMsembler explains model performance by highlighting relevant metabolic pathways and GPRs alternatives, informing experiments to resolve model uncertainty. Thus, GEMsembler facilitates building more accurate and biologically informed metabolic models for systems biology applications.