Pharmacological AMP-activated protein kinase activation suppresses low glucose-dependent macrophage migration inhibitory factor release from macrophages
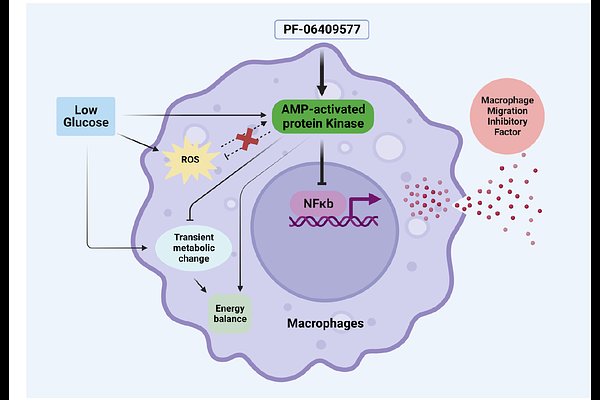
Pharmacological AMP-activated protein kinase activation suppresses low glucose-dependent macrophage migration inhibitory factor release from macrophages
Zhang, J.; Pollard, A. E.; Carling, D.; Benoit, V.; Ellacott, K. L.; Beall, C.
AbstractAims/hypothesis: Acute hypoglycemia promotes pro-inflammatory cytokine production, increasing risk for cardiovascular events in diabetes. AMP-activated protein kinase (AMPK) is regulated by and influences production of pro-inflammatory cytokines. We tested the mechanistic role of AMPK in low glucose induced changes in the pro-inflammatory cytokine macrophage migration inhibitory factor (MIF), which is elevated in patients with diabetes. Methods: Macrophage cell line Raw264.7 cells, primary macrophage bone marrow derived macrophages obtained from wild type mice or AMPK gamma 1 gain-of-function mice were utilized, as were AMPK alpha1/alpha2 knockout mouse embryonic fibroblasts (MEF). Allosteric AMPK activators PF-06409577 and BI-9774 were used, in conjunction with inhibitor SBI-0206965 were also used. We examined changes in protein phosphorylation/expression using western blotting, and protein localisation using immunofluorescence. Metabolic function was assessed using extracellular flux analyses and luciferase-based ATP assay. Cytokine release was quantified by ELISA. Oxidative stress was detected using a fluorescence-based ROS assay, and cell viability was examined using flow cytometry. Results: Macrophages exposed to low glucose showed a transient and modest activation of AMPK and a metabolic shift towards increased oxidative phosphorylation. Low glucose induced oxidative stress and increased release of macrophage migration inhibitory factor (MIF). Pharmacological activation of AMPK by PF-06409577 and BI-9774 attenuated low glucose-induced MIF release, with a similar trend noted with genetic activation using AMPK gamma 1 gain-of-function (D316A) mice, which produced a mild effect on low glucose-induced MIF release. Inhibition of NFkappaB signalling diminished MIF release and AMPK activation modestly but significantly reduced low glucose-induced nuclear translocation of NFkappaB. AMPK activation did not alter low glucose-induced oxidative stress in macrophages but application of AMPK inhibitor SBI-0206965 enhanced oxidative stress in macrophages and in AMPK knockout MEFs, suggesting an AMPK-independent mechanism Conclusions/interpretation Taken together, these data indicate that pharmacological AMPK activation suppresses release of MIF from macrophages. This is mediated by reduced activation of NFkappaB signalling in response to low glucose-induced oxidative stress and suggests that pharmacological AMPK activation could be a useful strategy for mitigating hypoglycemia-induced inflammation.