Adrenergic Hypersensitivity Drives Ventricular Arrhythmias Following Loss of Plexin-Mediated Cardiac Innervation
Adrenergic Hypersensitivity Drives Ventricular Arrhythmias Following Loss of Plexin-Mediated Cardiac Innervation
Zhu, C.; Lucero, E.; Patel, R.; Makita, T.; Wang, J. J.; Cao, Y.; Rockman, H. A.; Shivkumar, K.
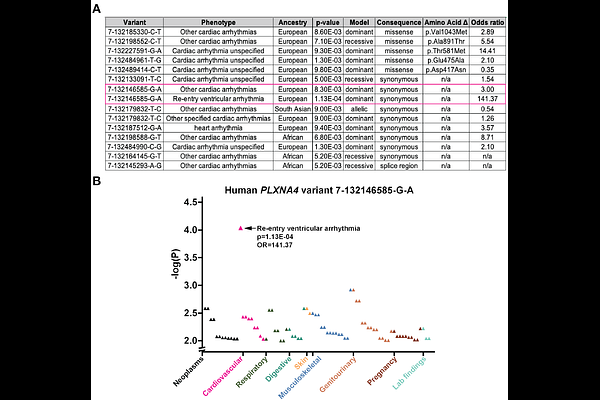
AbstractBackground: Ventricular arrhythmias (VAs) are a leading cause of death and arise from a combination of cardiac muscle injury and dysfunction of the intramyocardial sympathetic nerves that control cardiac electrophysiology. The adrenergic mechanisms by which intramyocardial nerves contribute to arrhythmogenesis are poorly understood. Semaphorin-plexin signaling pathways are responsible for developmental guidance of sympathetic nerves onto the heart and have previously been associated with VAs in humans. Objective: To investigate adrenergic control of arrhythmogenesis, we probed the cardiac electrophysiology of a Plexin-A3/-A4 double knockout mouse with loss of cardiac adrenergic nerves. Methods: We studied cardiac structure and function using tissue clearing, immunohistochemistry, and echocardiography. We utilized ECG in vivo and ex vivo in a Langendorff preparation to evaluate electrophysiologic responses to pharmacologic beta ({beta})-adrenergic stimulation and blockade, and we measured {beta}-adrenergic receptor ({beta}AR) density in cardiac membranes using radioligand binding. Finally, we performed a phenome-wide association study utilizing data from the UK Biobank to search for associations between PLXNA4 and human arrhythmias. Results: Mice with loss of plexin-dependent cardiac innervation had structurally normal hearts but displayed spontaneous VAs driven by adrenergic hypersensitivity, as well as increased cardiac {beta}AR density. Several human PLXNA4 variants were associated with arrhythmia phenotypes. Conclusion: These data establish a model of VAs driven purely by enhanced adrenergic receptor signaling, in the absence of structural heart disease, which can be used to investigate adrenergic mechanisms of arrhythmogenesis and to identify novel antiarrhythmic targets.