A Conserved Inhibitory Interdomain Interaction Regulates DNA-binding Activities of Hybrid Two-component Systems in Bacteroides
A Conserved Inhibitory Interdomain Interaction Regulates DNA-binding Activities of Hybrid Two-component Systems in Bacteroides
Gao, R.; Wu, T.; Stock, A. M.
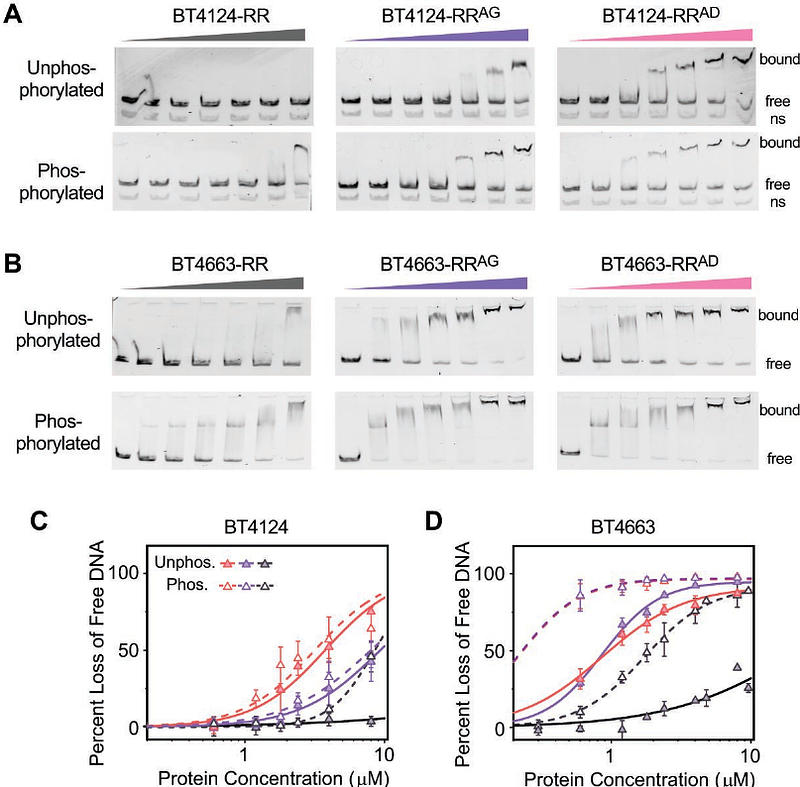
AbstractHybrid two-component systems (HTCSs) comprise a major class of transcription regulators of polysaccharide utilization genes in Bacteroides. Distinct from classical two-component systems in which signal transduction is carried out by intermolecular phosphotransfer between a histidine kinase (HK) and a cognate response regulator (RR), HTCSs contain the membrane sensor HK and the RR transcriptional regulator within a single polypeptide chain. Tethering the DNA-binding domain (DBD) of the RR with the dimeric HK domain in an HTCS could potentially promote dimerization of the DBDs and would thus require a mechanism to suppress DNA-binding activity in the absence of stimulus. Analysis of phosphorylation and DNA-binding activities of several HTCSs from Bacteroides thetaiotaomicron revealed a DBD-suppression mechanism in which an inhibitory interaction between the DBD and the phosphoryl group-accepting receiver domain (REC) decreases autophosphorylation rates of HTCS-RECs as well as represses DNA-binding activities in the absence of phosphorylation. Sequence analyses and structure predictions identified a highly conserved sequence motif correlated with a conserved inhibitory domain arrangement of REC and DBD. Presence of the motif, as in most HTCSs, or its absence, in a small subset of HTCSs, is likely predictive of two distinct regulatory mechanisms evolved for different glycans. Substitutions within the conserved motif relieve the inhibitory interaction and result in elevated DNA-binding activities in the absence of phosphorylation. Our data suggest a fundamental regulatory mechanism shared by most HTCSs to suppress DBD activities using a conserved inhibitory interdomain arrangement to overcome the challenge of the fused HK and RR components.
