A nanobody-enzyme fusion protein targeting PD-L1 and sialic acid exerts anti-tumor effects by affecting tumor associated macrophages
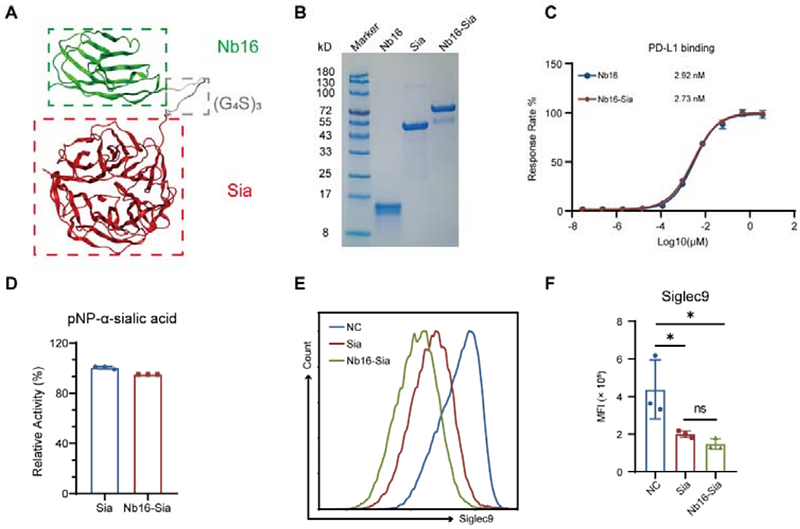
A nanobody-enzyme fusion protein targeting PD-L1 and sialic acid exerts anti-tumor effects by affecting tumor associated macrophages
Tong, Y.; Lu, X.; Chen, R.; Chen, C.; Sun, G.; Yu, X.; Lyu, S.; Feng, M.; Long, Y.; Gong, L.; CHEN, L.
AbstractCancer cells employ various mechanisms to evade immune surveillance. Their surface features, including a protective \"sugar coat\" and immune checkpoints like PD-L1 (programmed death ligand 1), can impede immune cell recognition. Sialic acids, which carry negative charges, may hinder cell contact through electrostatic repulsion, while PD-L1 transmits immunosuppressive signals to T cells. Furthermore, cancer cells manipulate macrophages within the tumor microenvironment to facilitate immune escape. Prior research has demonstrated the effectiveness of separately blocking the PD-L1 and sialic acid pathways in eliciting anti-tumor effects. In this study, we investigated the relationship between PD-L1 expression and genes associated with sialic acid in clinical databases. Subsequently, we developed a novel nanobody enzyme fusion protein termed Nb16-Sia to simultaneously target both PD-L1 and sialic acid pathways. In vivo experiments confirmed the anti-tumor activity of Nb16-Sia and highlighted its dependence on macrophages. Further investigations revealed that Nb16-Sia could polarize macrophages towards the M1 phenotype through the C-type lectin pathway in vitro and eliminate tumor-associated macrophages in vivo. In conclusion, our findings demonstrate that the fusion of PD-L1 nanobody with sialidase effectively targets tumor-associated macrophages, resulting in significant anti-tumor effects. This approach holds promise for drug development aimed at enhancing immune responses against cancer.


