Adenosine and acute low oxygen conditions suppress urinary bladder contractility through the activation of adenosine 2B receptors and large-conductance calcium-activated potassium channels
Adenosine and acute low oxygen conditions suppress urinary bladder contractility through the activation of adenosine 2B receptors and large-conductance calcium-activated potassium channels
Herrera, G. M.; Heppner, T. J.; Hennig, G. W.; Rengo, J. L.; Hepp, A. M.; Sancho, M.; Huerta de la Cruz, S.; Nelson, M. T.; Klug, N. R.
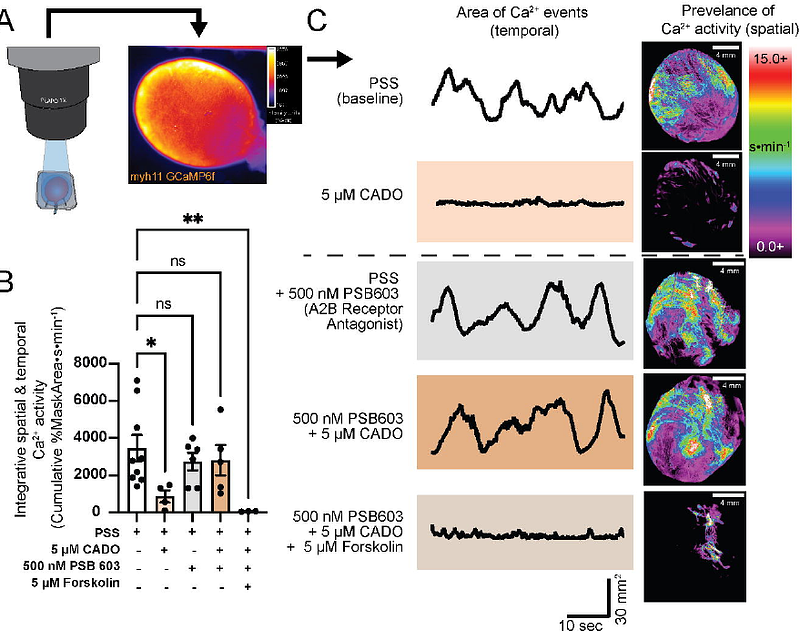
AbstractUnder healthy conditions the urinary bladder undergoes relatively long periods of filling with well-spaced voiding events to ensure proper storage and removal of urine respectively. During the filling phase, distinct contractile events in detrusor urinary smooth muscle (UBSM) elicit transient non-voiding pressure events and associated bursts in afferent nerve activity to relay the sensation of bladder fullness. The mechanisms that regulate UBSM excitability and associated non-voiding pressure events under physiological and pathological conditions are poorly understood. Here we investigated the role of adenosine signaling in regulating urinary bladder contractility. Using an ex vivo pressurized bladder preparation from mice and patch-clamp electrophysiology in isolated UBSM we evaluated whole bladder transient pressure events, whole bladder detrusor Ca2+ activity, and single UBSM ion channel activity. We found that adenosine suppresses bladder activity through activation of A2B adenosine receptors and downstream activation of large-conductance calcium-activated potassium (BKCa) channels. We further demonstrated that acute exposure to low oxygen conditions using a chemical oxygen scavenger potently suppresses bladder contractility through the A2B receptor pathway. These results highlight the prominent role adenosine receptors and downstream potassium channels play in regulating urinary bladder contractility in physiological and pathological contexts.