Enhancing PDAC Therapy: Decitabine-Olaparib Synergy Targets KRAS-Dependent Tumors
Enhancing PDAC Therapy: Decitabine-Olaparib Synergy Targets KRAS-Dependent Tumors
Anastasio, G.; Felaco, M.; Lamolinara, A.; Del Pizzo, F.; Cacciagrano, E.; Mottini, C.; Mutarelli, M.; Di Modugno, F.; Iezzi, M.; Cardone, L.
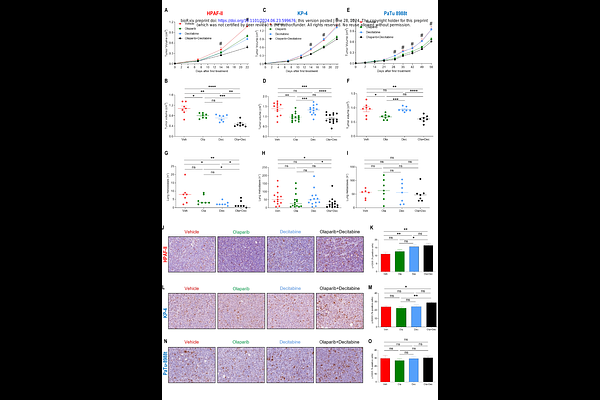
AbstractCurrent chemotherapies provide limited clinical benefits to patients with pancreatic ductal adenocarcinoma (PDAC), partly due to the lack of effective biomarkers for personalized therapy. KRAS activating mutations occur in almost 90% of PDAC cases, leading to a subset of tumors dependent on KRAS for survival (dKRAS). Assessing dKRAS in PDAC can be achieved using gene expression signature scores, independent of specific KRAS mutations, allowing for personalized therapies. Previous studies have shown that dKRAS-PDAC cells are more sensitive to the FDA-approved drug decitabine (DEC), although the mechanism remains unclear. While DEC is approved for hematological tumors, its repurposing in solid tumors poses challenges due to high-dose hematological side effects. Identifying optimal pharmacological approaches and response biomarkers is crucial for the successful clinical implementation of DEC in PDAC and other solid tumors. Our investigation revealed that low-dose DEC combined with the PARP inhibitor olaparib (OLA) enhances antitumor activity in dKRAS-PDAC. Mechanistically, DEC induces DNA damage and activates an ATR/ATM-mediated DNA damage response (DDR), with PARP1-mediated DNA repair playing a crucial role. Inhibiting PARP activity with OLA enhances antitumor activity, even in BRCA1/2wild-type and homologous recombination (HR)-proficient tumors, but it is ineffective in KRAS-independent tumors. Thus, transcriptomic-based KRAS dependency scores effectively predict the efficacy of combined therapy. Additionally, in dKRAS-PDAC tumors carrying a BRCA2 mutation, low-dose DEC enhances OLA\'s antitumor activity, completely inhibiting metastasis growth compared to single-drug treatments. Our findings support further clinical evaluation of DEC+OLA combination therapy in PDAC, particularly in dKRAS-positive tumors, irrespective of BRCA1/2 status. This approach extends the clinical benefit of OLA beyond BRCA status, addressing a limitation in targeted PARP inhibitor therapies. Furthermore, we highlight DDR as a key mechanism of action for DEC, beyond its role in gene expression regulation, underscoring the mode of action of this widely used anticancer drug.