Expanding the Repertoire of Photoswitchable Unnatural Amino Acids for Enzyme Engineering
Expanding the Repertoire of Photoswitchable Unnatural Amino Acids for Enzyme Engineering
Hiefinger, C.; Marcon, M.; Pape, V.; Casadevall, G.; Lahmy, R.; Nazet, J.; Bartl, M.; Bruckmann, A.; Osuna, S.; Koenig, B.; Hupfeld, A.
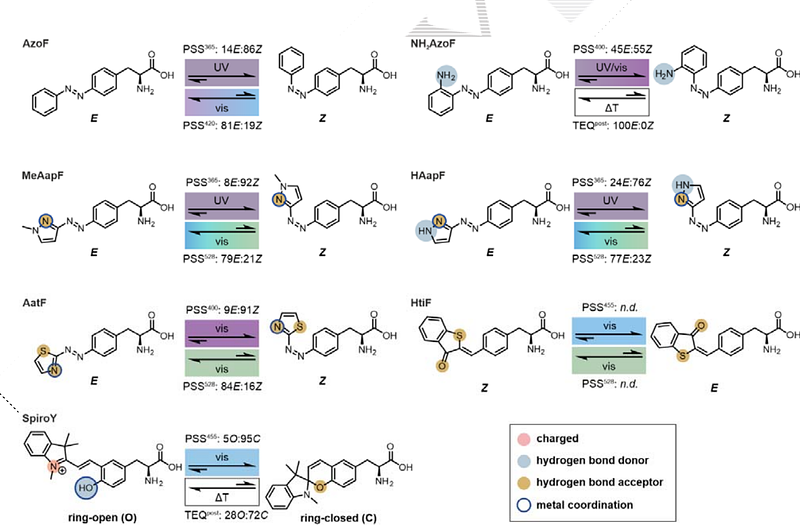
AbstractPhotoswitchable unnatural amino acids (psUAAs) play a crucial role in the engineering of light-sensitivity in enzymes, which holds significant promise for diverse applications such as biotherapy and biocatalysis. Besides near-quantitative photoconversion, the success and expediency of a psUAA for a certain application is defined by its interaction potential with the enzyme, its thermal stability and its effective wavelength of irradiation. To establish high versatility in the current repertoire, we have designed and synthesized six psUAAs based on azobenzene, arylazopyrazole, arylazothiazole, hemithioindigo and spiropyran photoswitches. The resulting psUAAs exhibit an enhanced interaction potential within an enzyme owing to their capacity for hydrogen bonding, ionic interactions, and metal ion coordination. Moreover, we observed diverse photochemical behaviors among the psUAAs, with four of them reversibly switching between the isomers with purely visible light. Notably, we identified orthogonal aminoacyl-tRNA synthetases that facilitate the incorporation of five of the six psUAAs co-translationally and computationally analyzed the synthetase-psUAA interactions. Finally, we evaluated the photochemical behavior of the five psUAAs within an enzymatic model and tested the photocontrol of catalysis confirming their diversity. Ultimately, our findings significantly expanded the repertoire of psUAAs and demonstrated their feasibility for enzyme engineering studies.