In vitro model of human subcutaneous adipocyte spheroids for studying mitochondrial dysfunction and mitochondria activating compounds
In vitro model of human subcutaneous adipocyte spheroids for studying mitochondrial dysfunction and mitochondria activating compounds
Wagner, A.; Lautaoja-Kivipelto, J.; Pehkonen, K.; Hassinen, A.; Kuusela, M.; Röttger, L.; Herbers, E.; Ioannidou, A.; Mädler, S.; Rothenaigner, I.; Srinivasan, S.; Laasonen, S.; Rahman, M. T.; Elomaa, P.; Kortetjärvi, S.; Olsson, A.; Ukkola, O.; Hadian, K.; Mann, M.; Peltoniemi, H.; Pietiläinen, K. H.; Klingenspor, M.; Virtanen, K. A.; Hagberg, C. E.; Pirinen, E.
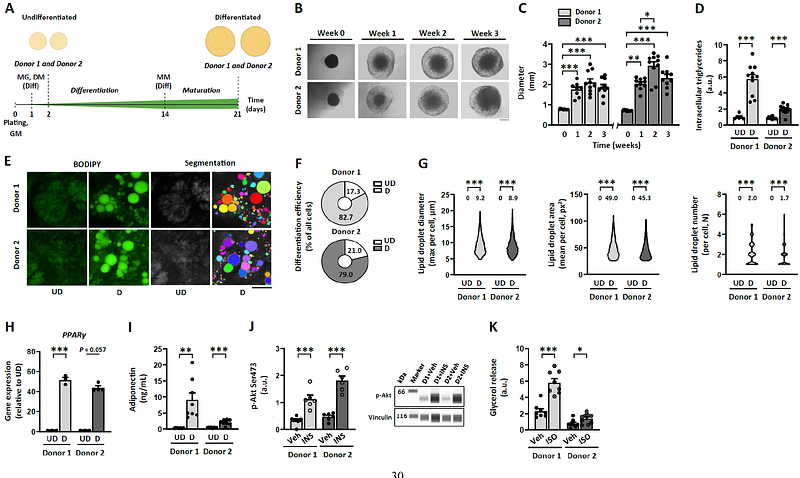
AbstractMitochondrial abnormalities drive subcutaneous white adipose tissue dysfunction in obesity, leading to metabolic complications. A key challenge in obesity research is the lack of in vitro models to study human adipocyte mitochondria. Here, we establish a human subcutaneous adipocyte spheroid model to characterize mitochondrial metabolism under obesity-relevant conditions and drug exposure. Human preadipocyte spheroids were differentiated in ultra-low attachment plates for 3 weeks in media free of thiazolidinediones. The differentiated adipocyte spheroids showed increased lipid accumulation, adipogenic gene expression, mitochondrial respiration and adiponectin secretion, and a proper response to hormonal stimulation. Lipid mixture administration during differentiation induced metabolic disturbances including mitochondrial respiration failure associated with increased mitochondrial biogenesis. Post-differentiation treatment with rosiglitazone, a peroxisome proliferator-activated receptor gamma agonist, improved mitochondrial bioenergetics and adiponectin secretion in lipid mixture-administered adipocyte spheroids.