Studies of all-trans retinoic acid transport in myopigenesis
Studies of all-trans retinoic acid transport in myopigenesis
Chatterjee, S.; Roy, A.; Yu, J.; Read, A. T.; Bentley-Ford, M. R.; Pardue, M. T.; Kane, M. A.; Finn, M. G.; Ethier, C. R.
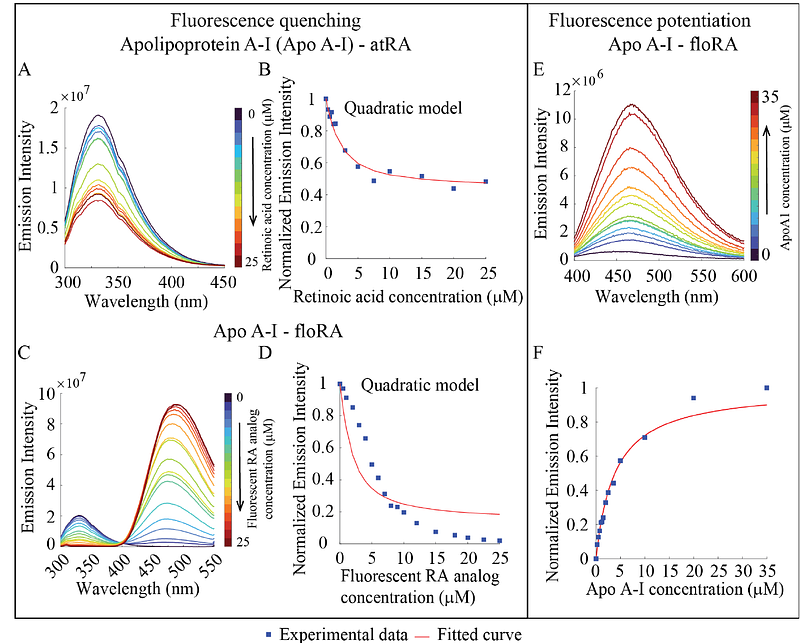
AbstractPurpose: Myopia incidence is increasing globally. All-trans retinoic acid (atRA) is important in myopigenic retinoscleral signaling, motivating research on its ocular transport. However, atRA\'s weak autofluorescence limits its direct visualization in tissues. Further, atRA is hydrophobic and must bind to protein carriers for transport. We assessed a fluorescent analog of atRA (LightOx14, CAS:198696-03-6, referred as \'floRA\'), as an experimentally accessible atRA surrogate by: (i) evaluating its binding to carrier proteins and (ii) visualizing its distribution in ocular tissues. Methods: Binding: We assessed atRA-carrier protein binding using fluorescence quenching assays with bovine serum albumin (BSA), high density lipoprotein (HDL), apolipoprotein A-I (Apo A-I) and retinol binding protein 4 (RBP4). Direct visualization: Wild-type C57BL/6J mice were euthanized, eyes enucleated, and wedges containing sclera and choroid incubated for specific durations in 50 M floRA+BSA. The wedge centers were cryo-sectioned and counterstained for nuclei. Fluorescent micrographs were acquired and analyzed using ImageJ. Results: Association constants (Ka) for atRA and floRA binding to carrier proteins were similar and ranged from 2-13 x 105 M-1, indicating non-specific binding. floRA could be visualized in sclera and choroid, yet showed significant spatial heterogeneity (enhanced fluorescence often colocalizing with nuclei). Conclusions: floRA is a reasonable surrogate for atRA binding to BSA, HDL, Apo A-I and RBP4. Considering these proteins\' relative serum and extravascular abundances, and their similar binding affinity to atRA, we predict that serum albumin is an important atRA carrier. Use of floRA in whole tissue tracer studies shows promise but requires further refinement.