GM-CSF and IL-3 expression increases immune engraftment and tumor infiltration in a humanized patient-derived xenograft model of hepatocellular carcinoma
GM-CSF and IL-3 expression increases immune engraftment and tumor infiltration in a humanized patient-derived xenograft model of hepatocellular carcinoma
Weinfurtner, K.; Tischfield, D.; Crainic, J.; Li, W.; Kaplan, D. E.; Gade, T. P.
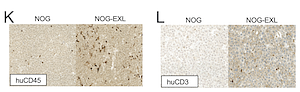
AbstractIntroduction: Immunotherapy has shown promising results in advanced hepatocellular carcinoma (HCC), and relevant model systems are greatly needed to inform treatment paradigms. Transplantation of immunodeficient mice with human hematopoietic cells allows for the development of humanized patient derived xenografts (HIS PDXs); however, these models have limited development of myeloid lineages. We aimed to determine the impact of human GM-CSF and IL-3 expression on tumor immune cell infiltration and tumor growth in a HIS PDX model of HCC. Materials and Methods: HIS HCC PDXs were generated using NOG (NOD/Shi-scid/IL-2R{gamma}null) and NOG-EXL (huGM-CSF/huIL-3 NOG) mice conditioned with 30mg/kg Busulfan and, 24 hours later, injected with 400,000 CD34+ cells isolated from human fetal livers. HCC tumor tissue from an established PDX line in Matrigel was then implanted subcutaneously (SQ). Immune engraftment was monitored by flow cytometry. Mice were sacrificed when tumors reached 2cm and tumor, blood, liver, and spleen were analyzed by flow cytometry. Results: HIS NOG-EXL HCC mice demonstrated earlier and persistently increased huCD45+ peripheral blood immune cells compared to HIS NOG HCC mice with 12.1% vs 1.7% at tumor implantation (p<0.0001) and 82.1% vs 43.8% at steady state (p<0.0001). All major immune cell types were represented in both groups. There was no difference in tumor growth between HIS NOG HCC, HIS NOG-EXL HCC, and control NOG HCC tumors by latency (48.4, 45.5, and 50.5 days, respectively, p=0.63) or Gompertzian growth rates (0.32, 0.31, 0.40, respectively, p=0.68). At necropsy, HIS NOG-EXL HCC mice had increased huCD45+ immune cells in tumor (27.2% vs 8.6%, p=0.03) compared to HIS NOG HCC mice with increased CD4+ regulatory T cells (21.0% vs 11.1%, p=0.04), CD4+ T cell PD-1 expression (77.9% vs 49.4%, p=0.03), and M2 macrophage phenotype (36.1% vs 17.6%, p=0.04). Conclusions: HIS HCC PDX models demonstrate robust immune infiltration in the peripheral blood, spleen, liver, and SQ HCC tumor, especially in mice expressing human GM-CSF and IL-3. Expression of these human cytokines lead to increased tumor infiltrating immune cells with a higher proportion of regulatory immune cells, suggesting NOG-EXL mice may be a more appropriate model for preclinical trials with immunotherapy.