Assessing mechanisms driving phenol isopropylated phosphate (IPP)- induced larval photomotor response deficits in zebrafish
Assessing mechanisms driving phenol isopropylated phosphate (IPP)- induced larval photomotor response deficits in zebrafish
Sharma, S.; Rojas, A.; Serradimigni, R.; Leong, C.; Dasgupta, S.
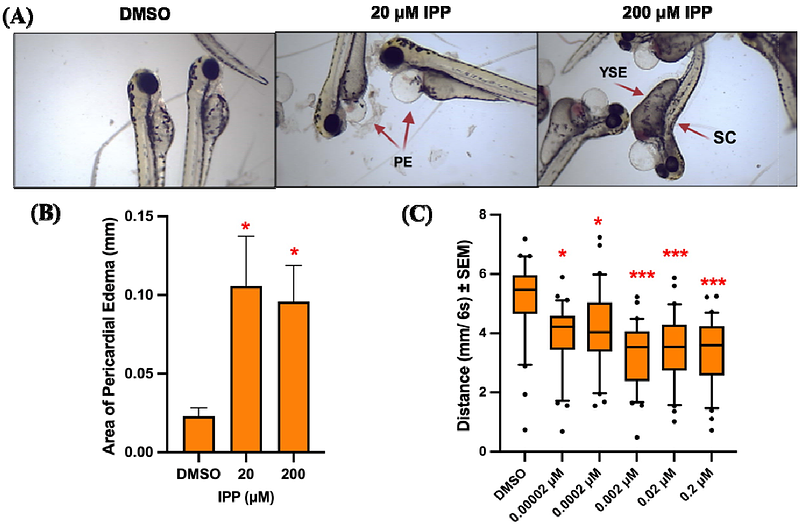
AbstractPhenol isopropylated phosphates (IPP) are an additive organophosphate flame retardant (OPFR) which has been extensively used in furniture, electronics, automobiles, plastics, and childrens products to slow down the spread of fire. The processing and distribution of IPP- containing products have been prohibited but its continuous leaching from end use products has retained the concern of its toxicity. The present study was designed to evaluate IPP-induced developmental toxicity using zebrafish embryos. We first conducted range finding experiments with embryonic zebrafish exposures to 0- 200 micromolar IPP from 6 to 120 h post fertilization and found significant morphological impacts like pericardial edema, yolk sac edema and spinal curvature at higher concentrations. For behavioral readouts, we performed larval photomotor response (LPR) assay at sublethal concentrations and observed hypoactive locomotory behavior in exposed larvae. Following this, relying on secondary analyses of our whole embryo mRNA-seq data, we conducted- 1) retinoic acid receptor (RAR) signaling assay and 2) DNA methylation assays. In vitro assay for RA receptors indicate that IPP significantly inhibits RAR-alpha;, but not RAR-beta; and RAR-gamma;. Whole-mount immunohistochemistry for 5-methylcytosine and global DNA methylation assay showed significant IPP-induced hypermethylation in situ. We conducted IPP co-exposure studies with a methylome modifier 5-azacytidine (Aza-c a methylation inhibitor) or retinoic acid signaling activators to assess if LPR phenotypes were mitigated by co-exposures. Data showed that Aza-c co-exposures partially reversed IPP-induced LPR hypoactivity and DNA hypermethylation, co-exposure with retinoic acid as well as AM580 (an RAR alpha; activator) were not able to reverse IPP-induced hypoactivity. Finally, based on RNA-seq data, we hypothesized that IPP affects the development of brain and eyes. Firstly, we performed global DNA methylation in brain and eyes, but did not find any significant effects. Then, we conducted mRNA sequencing on dissected brains and eyes, and found 2 and 135 differentially expressed genes, respectively. Gene ontology revealed that IPP affect phototransduction, voltage gated ion channels, synaptic and neurotransmitter signaling. Collectively, our data shows that IPP induces morphological abnormalities and disrupts larval photo motor response, potentially through methylomic regulation. Finally, we observed that IPP affects gene expression within the developing eye, establishing synaptic transmission, vision and muscle contraction as a potential causative factor for LPR responses.


