Resolving the pharmacological redox-sensitivity of SARS-CoV-2 PLpro in drug repurposing screening enabled identification of the competitive GRL-0617 binding site inhibitor CPI-169
Resolving the pharmacological redox-sensitivity of SARS-CoV-2 PLpro in drug repurposing screening enabled identification of the competitive GRL-0617 binding site inhibitor CPI-169
Kuzikov, M.; Morasso, S.; Reinshagen, J.; Wolf, M.; Monaco, V.; Cozzolino, F.; Grdadolnik, S. G.; Sket, P.; Plavec, J.; Iaconis, D.; Summa, V.; Esposito, F.; Tramontano, E.; Monti, M.; Beccari, A. R.; Windshugel, B.; Gribbon, P.; Storici, P.; Zaliani, A.
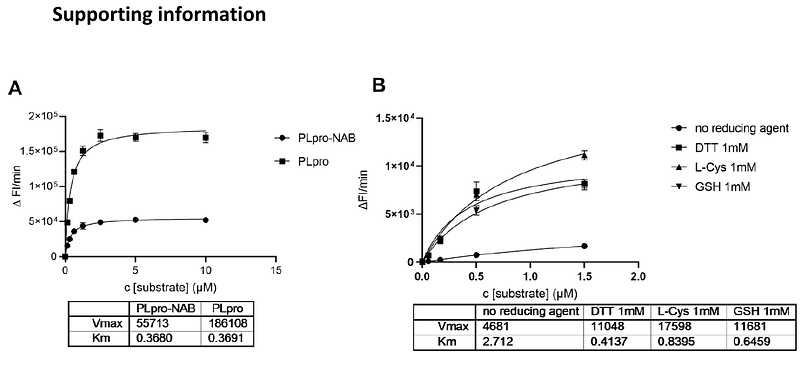
AbstractThe SARS CoV-2 Papain-Like protease has multiple roles in the viral replication cycle, related to both its polypeptide cleavage function and its capacity to antagonize host immune response. Targeting PLpro function is recognized as a promising mechanism to modulate viral replication whilst supporting host immune responses. However, development of PLpro specific inhibitors remains challenging. Upcoming studies revealed the limitation of reported inhibitors by profiling them through a pipeline of enzymatic, binding and cellular activity assays showing unspecific activity. GRL-0617 remained the only validated molecule with demonstrated anti-viral activity in cells. In this study we refer to the pitfalls of redox-sensitivity of PLpro. Using a screening-based approach to identify inhibitors of PLpro proteolytic activity, we made extensive efforts to validate the active compounds over a range of conditions and readouts, emphasising the need for comprehensive orthogonal data when profiling putative PLpro inhibitors. The remaining active compound CPI-169, showed to compete with GRL-0617 in NMR-based experiments, suggesting to share a similar binding mode, opening novel design opportunities for further developments as antiviral agents.