Selective Detection of a Key Region in Chemotaxis Signaling Protein Complexes by Solid-State NMR
Selective Detection of a Key Region in Chemotaxis Signaling Protein Complexes by Solid-State NMR
Allen, J. J.; Wang, S.; Rienstra, C. M.; Thompson, L. K.
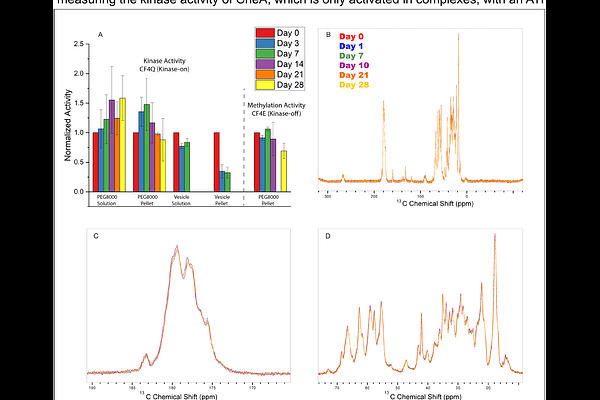
AbstractUnderstanding how large protein complexes function requires tools that can resolve both structure and dynamics in their native assembly states. Here, we apply solid-state NMR (SSNMR) to selectively detect rigid regions of a receptor protein fragment in the context of the >500 kDa chemoreceptor signaling complex found in chemotactic bacteria. These complexes assemble into hexagonal arrays that network multiple active units together. The cytoplasmic fragment of the E. coli aspartate chemoreceptor exhibits dynamics on multiple timescales across different regions of the protein, and these dynamics differ between signaling states. We apply 13C-15N dipolar coupling-based SSNMR experiments to selectively probe the rigid portion (motions slower than millisecond timescale) of this protein, in the context of the full array structure. We optimized assembly methods to form native-like, homogeneous complexes capable of maintaining activity and sample integrity during extended NMR experiments with low electric-field NMR probe designs. A subset of the protein, approximately 100 residues at the membrane-distal tip of the chemoreceptor where it interacts with its associated kinase, was detected and identified as the most rigid region. Chemical shift changes for many residues in this rigid region were observed between NMR spectra of the kinase-on and kinase-off signaling states. This suggests conformational changes occur at the chemoreceptor tip during signaling, which have not been observed in previous studies of this system. These findings demonstrate a dynamics-based NMR spectral editing approach to selectively examine a key region of a large signaling protein within its macromolecular assembly.