A Catalytically Inactive Protein Kinase C alpha Mutation Drives Chordoid Glioma by Pathway Rewiring
A Catalytically Inactive Protein Kinase C alpha Mutation Drives Chordoid Glioma by Pathway Rewiring
Bellamy, C.; Tovell, H.; Schwaighofer, S.; Baffi, T.; Arslan, J.; Letourneur, Q.; Dingli, F.; Loew, D.; Kornev, A.; Lerond, J.; Kao, T.; Liva, S.; Izac, B.; Andrieu, M.; Adle-Biassette, H.; Barnier, J.-V.; Stefan, E.; Sanson, M.; Newton, A.; Bielle, F.
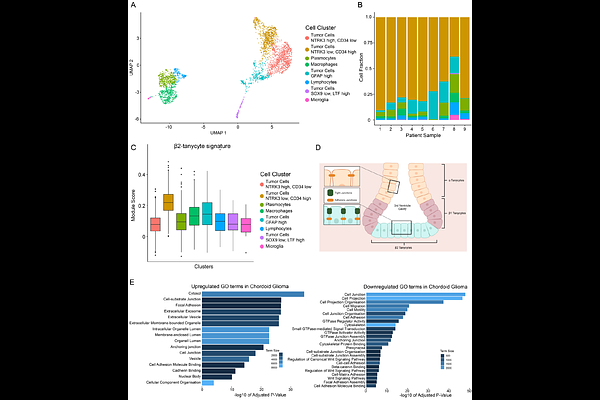
AbstractChordoid glioma (ChG) is a rare, low-grade brain tumor characterized by a novel recurrent point mutation, D463H, in the kinase domain of protein kinase C alpha (PKC). The mutation is invariably an Asp to His substitution, suggesting it endows a unique function beyond catalytic inactivation associated with other cancer-associated PKC mutations. Here we use in vitro and in cellulo activity assays to show that PKCD463H is catalytically inactive, functions as a dominant-negative mutant to suppress endogenous PKC and uniquely rewires the cellular interactome. Specifically, phosphoproteomic, proximity labeling, and co-immunoprecipitation mass-spectrometry data from cells overexpressing PKCD463H identify altered phosphorylation of substrates and binding to multiple proteins involved in cell-cell junctions compared to WT enzyme. Lastly, single nuclei RNAseq reveals that ChG derives from specialized tanycytes. Our data suggest that this disease-defining, fully penetrant mutation promotes neomorphic non-catalytic scaffolding to impair cell junction function.