Inclusion of Hsp70 co-chaperone and RNA binding activities facilitates optimal function of spliceosomal disassembly factor Cwf23 in intron-rich Schizosaccharomyces pombe
Inclusion of Hsp70 co-chaperone and RNA binding activities facilitates optimal function of spliceosomal disassembly factor Cwf23 in intron-rich Schizosaccharomyces pombe
Yadav, K.; Raut, S.; Guha Roy, M.; Sasikumar, G.; Desai, M.; R Pillai, B.; Kumar Mishra, S.; Sahi, C.
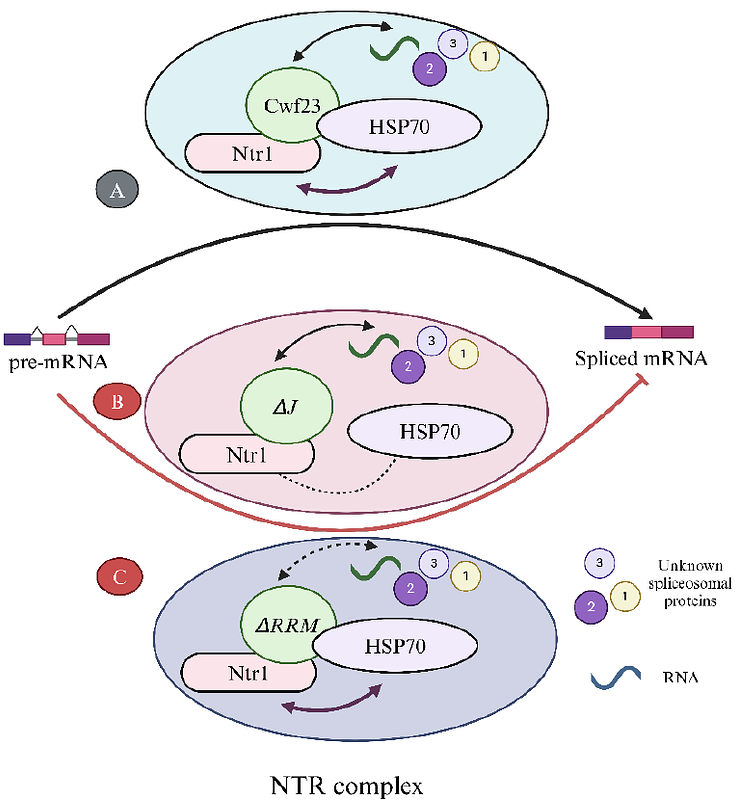
AbstractCwc23 is an essential J-domain protein that collaborates with the disassembly factor Ntr1 to facilitate spliceosomal disassembly. Although Cwc23 orthologs have been identified in spliceosomal extracts of many eukaryotes, their functionality in intron-rich eukaryotes remains largely unexplored. Here, we investigate the functionality of Cwf23, an ortholog of Cwc23 in Schizosaccharomyces pombe. Our study reveals that while the interaction between Cwf23 and SpNtr1 is conserved, it is not essential in S. pombe. Additionally, the RNA recognition motif (RRM) in Cwf23 is crucial for its function, as mutations in the RRM affect both growth and pre-mRNA splicing. Unlike its budding yeast counterpart, the J-domain of Cwf23 is indispensable, as J-domain mutants cannot support cell viability. These findings suggest a new paradigm in which the presence of a functional RRM and the essential nature of the J-domain underscore the increased requirements for an RNA-binding Hsp70 co-chaperone machinery in spliceosomal remodelling in intron-rich eukaryotes.


