Functional and structural analysis of a cyclization domain in a cyclic beta-1,2-glucan synthase
Functional and structural analysis of a cyclization domain in a cyclic beta-1,2-glucan synthase
Tanaka, N.; Saito, R.; Kobayashi, K.; Nakai, H.; Kamo, S.; Kuramochi, K.; Taguchi, H.; Nakajima, M.; Masaike, T.
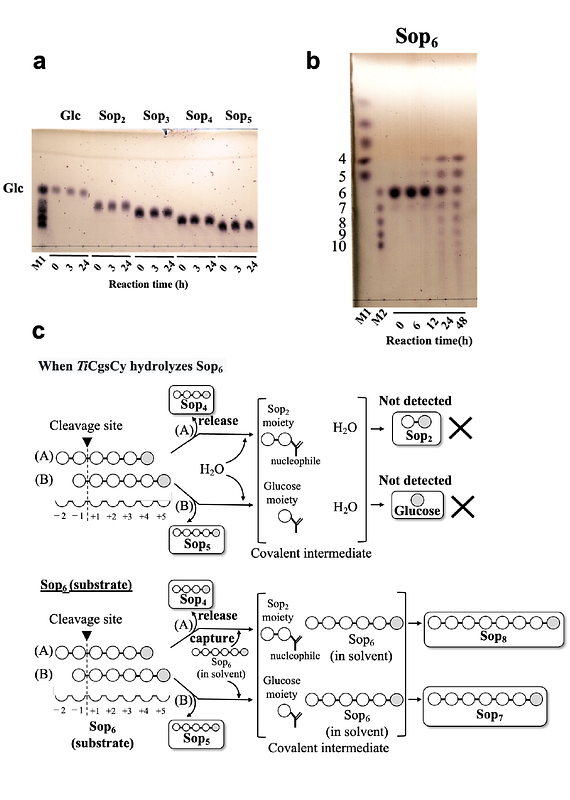
AbstractCyclic beta-1,2-glucan synthase (CGS) is a key enzyme in production of cyclic beta-1,2-glucans (CbetaGs) which are involved in bacterial infection or symbiosis to host organisms. Nevertheless, a mechanism of cyclization, the final step in the CGS reaction, has not been fully understood. Here we performed functional and structural analyses of the cyclization domain of CGS alone from Thermoanaerobacter italicus (TiCGSCy). We first found that beta-glucosidase-resistant compounds are produced by TiCGSCy with linear beta-1,2-glucans as substrates. The 1H-NMR analysis revealed that these products are CbetaGs. Next, action pattern analyses using beta-1,2-glucooligosaccharides revealed a unique reaction pattern: exclusive transglycosylation without hydrolysis and a hexasaccharide as the minimum length of the substrate. Nevertheless, longer substrate beta-1,2-glucooligosaccharides are preferred, being consistent with the fact that CGSs generally produce CbetaGs with degrees of polymerization of around 20. Finally, the overall structure of the cyclization domain of TiCGSCy was found to be similar to those of beta-1,2-glucanases in phylogenetically different groups. Meanwhile, the identified catalytic residues indicated clear difference in the reaction pathways between these enzymes. Therefore, we propose a novel reaction mechanism of TiCGSCy. Thus, we propose that the present group of CGSs should be defined as a new glycoside hydrolase family, GHxxx.