POU5F1B is a human-restricted ROCK inhibitor-sensitive oncoprotein that restructures membrane nanodomains to increase cell adhesion
POU5F1B is a human-restricted ROCK inhibitor-sensitive oncoprotein that restructures membrane nanodomains to increase cell adhesion
Simo Riudalbas, L.; Offner, S.; Abrami, L.; Unterauer, E. M.; Duc, J.; Planet, E.; Jungmann, R.; van der Goot, G.; Trono, D.
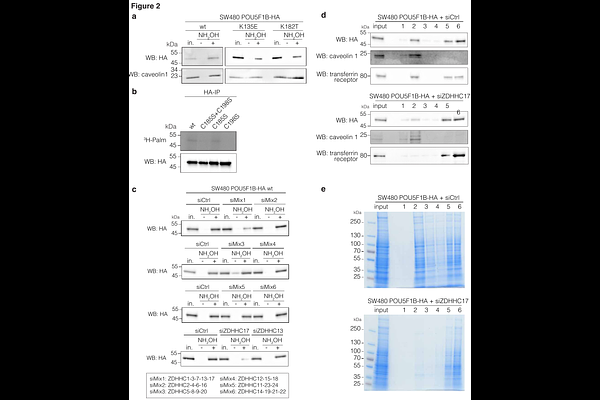
AbstractEvolution confers new species with distinctive biological features, which can translate in purely mechanistic speciation or in novel phenotypic traits. POU5F1B arose by retrotransposition of POU5F1/OCT4 in the last common ancestor of modern Hominoidae. Its human product is endowed with oncogenic properties, notably promoting gastrointestinal cancer growth and metastasis. Here, we reveal that the oncogenic action of POU5F1B requires ubiquitination of lysine residues found only in the human protein. This post-translational modification is essential for POU5F1B to localize to the cytoplasm and become acylated by the DHHC17 palmitoyltransferase. This leads to POU5F1B association with detergent-resistant membrane subdomains, where it triggers the accumulation of integrins and signaling molecules, and to a stimulation of cell focal adhesion. Finally, we determined that POU5F1B stability is critically dependent on ROCK, a crucial regulator of cytoskeletal dynamics and cell motility.