Insights from aquaporin structures into drug-resistant sleeping sickness
Insights from aquaporin structures into drug-resistant sleeping sickness
Matusevicius, M.; Corey, R. A.; Gragera, M.; Yamashita, K.; Sprenger, T.; Ungogo, M.; Blaza, J. N.; Castro-Hartmann, P.; Chirgadze, D. Y.; Chaitanya Vedithi, S.; Afanasyev, P.; Melero, R.; Warshamanage, R.; Gusach, A.; Carazo, J.-M.; Carrington, M.; Blundell, T.; Murshudov, G. N.; Stansfeld, P. J.; Sansom, M. S.; De Koning, H. P.; Tate, C. G.; Weyand, S.
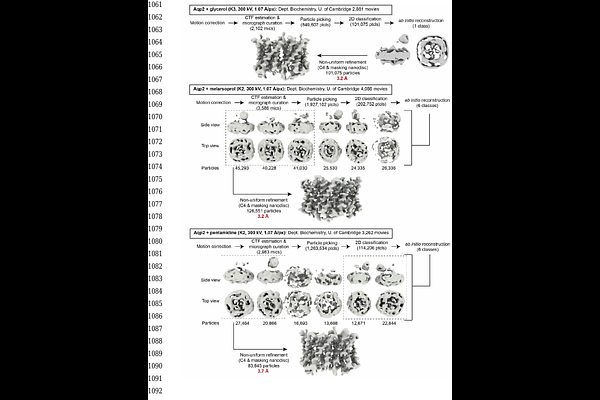
AbstractTrypanosoma brucei is the causal agent of African trypanosomiasis in humans and animals, the latter resulting in significant negative economic impacts in afflicted areas of the world. Resistance has arisen to the trypanocidal drugs pentamidine and melarsoprol through mutations in the aquaglyceroporin TbAQP2 that prevent their uptake. Here we use cryogenic electron microscopy to determine the structure of TbAQP2 from Trypanosoma brucei, bound to either the substrate glycerol or to the sleeping sickness drugs, pentamidine or melarsoprol. The drugs bind within the AQP2 channel at a site completely overlapping that of glycerol. Mutations leading to a drug-resistant phenotype were found in the channel lining. Molecular dynamics simulations showed the channel can be traversed by pentamidine, with a low energy binding site at the centre of the channel, flanked by regions of high energy association at the extracellular and intracellular ends. Drug-resistant TbAQP2 mutants still bind pentamidine, but the much weaker binding in the centre of the channel is insufficient to compensate for the high energy processes of ingress and egress, hence impairing transport at pharmacologically relevant concentrations. These structures of an aquaporin bound to a drug and represent a novel paradigm for drug-transporter interactions and could provide new mechanisms for targeting drugs into other pathogens or human cells.