Integrating process, proximity, and prediction implicates novel protein and RNA interactions in human Origin Recognition Complex function
Integrating process, proximity, and prediction implicates novel protein and RNA interactions in human Origin Recognition Complex function
Smolka, J. A.
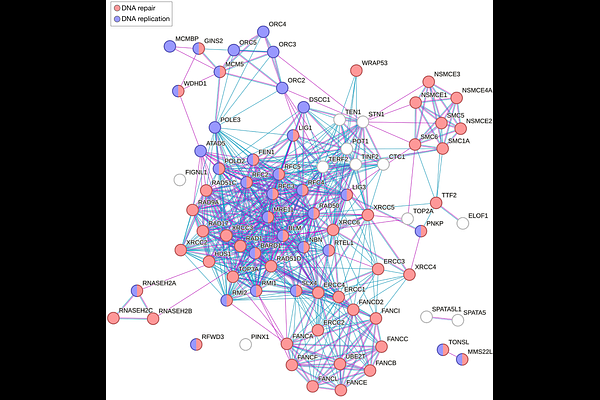
AbstractGenome replication start sites, called origins, begin to be specified by Origin Recognition Complex (ORC) proteins prior to replication through a process called origin licensing. Once licensed, origins become active and initiate DNA synthesis with varying efficiencies influenced by local chromatin environment, transcription, and 3D genome organization. ORC proteins have also been implicated in regulating chromatin state and nuclear organization. However, it is unclear if there is interplay between ORC and the chromatin architectures underlying origin activation, as we lack a systems-level understanding of how ORC proteins interact, post-licensing, with the nuclear environments conducive to genome synthesis. To infer this context-specific ORC interactome, I used data from genome-wide CRISPR fitness screens, a cell-wide proximity labeling study, and proteomic profiling of nascent DNA-associated proteins to identify 17 novel factors that genetically and proteomically interact with ORC subunits and genome replication. Unexpectedly, the candidate pool was significantly enriched for factors involved in the homeostasis of RNA Polymerase III (Pol III) transcripts, particularly 5S ribosomal RNA (rRNA) and transfer RNA (tRNA). Follow-up protein-protein structure predictions by AlphaFold 3 (AF3) proposed direct interactions between ORC subunits and Pol III transcript biogenesis factors, as well as epigenetic regulators and a cyclin-dependent kinase. Given the prominence of 5S rRNA and tRNA biogenesis factors in my results, and prior reports of ORC subunits binding RNA, I also used protein-RNA structure prediction to identify candidate ORC3 interactions with 5S rRNA and tRNA. Altogether, my analysis integrates biological process, molecular proximity in human cells, and structural prediction to nominate novel protein and RNA interactions for involvement in the human replication program. These results augment and expand current models for ORC function and origin activation, particularly those involving chromatin state and transcriptional activity, and generate testable hypotheses to explore the interdependencies of replication patterning, histone modification, and nuclear RNA homeostasis.