An Fc-SPINK1 fusion protein inhibits pancreatic inflammation in a mouse model
An Fc-SPINK1 fusion protein inhibits pancreatic inflammation in a mouse model
Way, J. C.; Heid, D.; Theiss, M.; Noerrelykke, S.; Barck, L.; Burger, L.; Bredl, J. A.; Dobbertin, T.; Hoeflich, P.; Stadelmann, T.; Niopek, D.; Chan, K. R.; Graveline, A.; Vernet, A.; Sanchez-Ventura, M.; Silver, P. A.
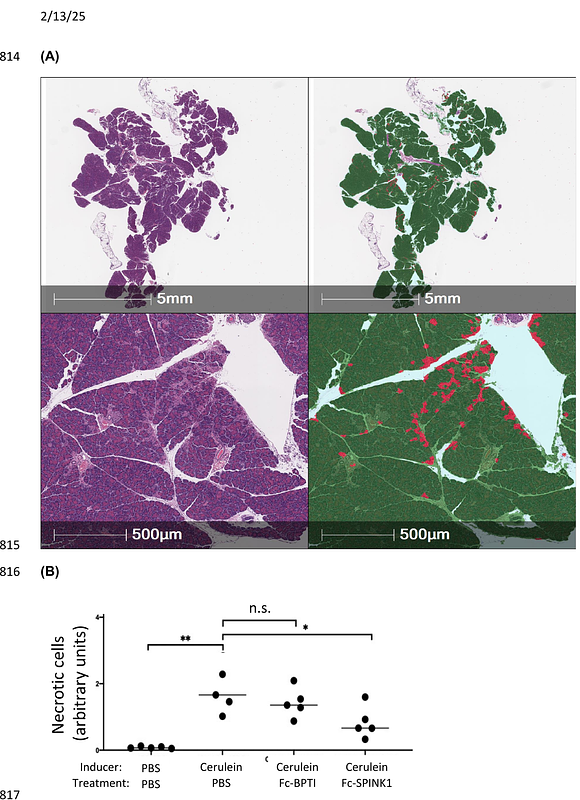
AbstractPancreatitis results from premature activation and impaired inactivation of pancreatic proteases, primarily trypsin, leading to self-digestion, tissue necrosis, fibrosis, and inflammation. SPINK1 is a pancreas-specific inhibitor of trypsin that prevents premature trypsin activation, and could be a candidate therapeutic. However, because of its small size, SPINK1 would be subject to rapid renal clearance, making it ineffective. To construct a long half-life therapeutic inhibitor of trypsin for pancreatitis treatment we fused this protein to the C-terminus of an IgG1 antibody Fc element, increasing the size to ~78 kDa, thereby exceeding the renal clearance threshold and providing for FcRn-mediated recycling out of cells. A non-glycosylated form of Fc-SPINK1 was expressed in the yeast Pichia pastoris. Fc-SPINK1 inhibits trypsin enzyme activity in vitro. The blood pharmacokinetics in mice are consistent with a three-compartment distribution model and a terminal half-life of ~3 days. In a caerulein-induced mouse model of pancreatitis, Fc-SPINK1 significantly ameliorated cell death and immune cell infiltration. We developed an automated image analysis technique to quantify pancreatitis-associated loss of tissue cohesion, and found that Fc-SPINK1 also reduced this effect. This study demonstrates the potential of Fc-SPINK1 as a rationally designed therapy for pancreatitis.