Integrated forward and reverse degradomics uncovers the proteolytic landscape of aortic aneurysms and the roles of MMP9 and mast cell chymase
Integrated forward and reverse degradomics uncovers the proteolytic landscape of aortic aneurysms and the roles of MMP9 and mast cell chymase
Martin, D. S.; Bhutada, S.; Cikach, F. S.; Neto, E. G.; Willard, B.; Ramkhelawon, B.; Chung, M. K.; Dahal, S.; Ramamurthi, A.; Joshi, J. P.; Blankenburg, D.; Barnard, J.; Blackstone, E. H.; Roselli, E. E.; Apte, S.
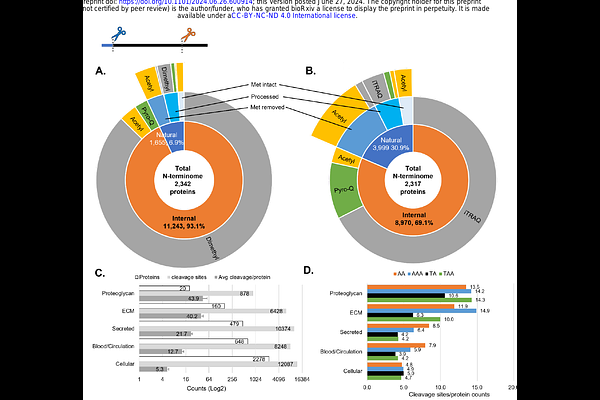
AbstractBackground: Dysregulated proteolysis is implicated in thoracic (TAA) and abdominal aortic aneurysm (AAA) pathogenesis, but the proteolytic landscapes (degradomes) of aneurysmal and normal aorta, and contributions of individual proteases remain undefined. Here, a proteome-wide approach was used to uncover TAA and AAA degradomes, compare them quantitatively and define the specific role in aortic remodeling of two proteases consistently identified in the aneurysms, mast cell chymase (CMA1) and matrix metalloprotease 9 (MMP9). Methods: The mass spectrometry-based N-terminomics strategy Terminal Amine Isotopic Labeling of Substrates (TAILS) was applied to Marfan syndrome TAAs (n=5), AAAs (n=16) and corresponding non-diseased aorta (TAs, n=4, and AAs, n=8) as a forward degradomics application, i.e., to define substrate and protease degradomes, and 8-plex iTRAQ-TAILS was used for quantitative comparison. Cleavage sites of CMA1 and MMP9 were sought by reverse degradomics, i.e., digestion of aortic proteins with these proteases, followed by 6-plex iTRAQ-TAILS. CMA1 and MMP9 proteolysis of biglycan was investigated using Amino-Terminal Oriented Mass spectrometry of Substrates (ATOMS). Results: We experimentally annotated 16,923 proteolytically derived peptides (substrate degradome) and 90 proteases (protease degradome) in the aorta. Quantitative substrate degradome comparisons identified specific differentially modulated pathways and networks in TAA and AAA. Reverse degradomics elucidated > 300 CMA1 and MMP9 substrate cleavage sites, of which, many, including orthogonally validated biglycan cleavage, occurred in the disease degradomes. Conclusions: Unbiased, proteome-wide forward degradomics of the aortic wall from TAA, AAA and non-diseased tissue generated the first systems biology view of vascular wall breakdown and public resource for the hitherto occult proteolytic landscape, demonstrating widespread extracellular matrix remodeling. The findings provide insights on aortic aneurysm pathways and potential disease biomarkers. Mapping of specific contributions of CMA1 and MMP9 on the aortic forward substrate degradome using reverse degradomics provides a strategy for defining the activities of all proteases involved in aortic disease.