Mechanism of SK2 channel gating and its modulation by the bee toxin apamin and small molecules
Mechanism of SK2 channel gating and its modulation by the bee toxin apamin and small molecules
Cassell, S. J.; Li, W.; Krautwald, S.; Khoshouei, M.; Lee, Y. T.; Hou, J.; Guan, W.; Peukert, S.; Weihofen, W.; Whicher, J.
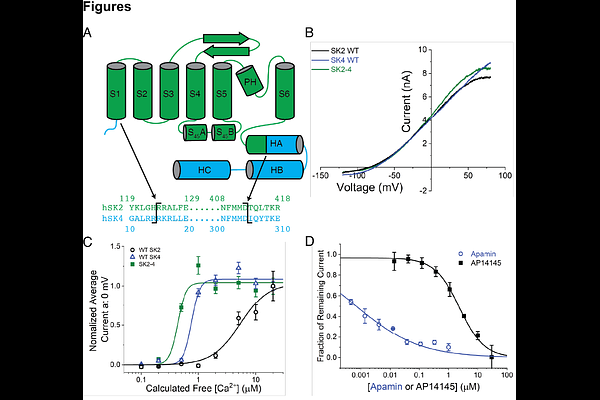
AbstractSmall-conductance calcium-activated potassium channel 2 (SK2) serves a variety of biological functions by coupling intracellular calcium dynamics with membrane potential. SK2 modulators are in development for the treatment of neurological and cardiovascular diseases, though the mechanisms of pharmacological modulation remain incompletely understood. We determined structures of an SK2-4 chimeric channel in Ca2+-bound and Ca2+-free conformations and in complex with the bee toxin apamin, a small molecule inhibitor, and a small molecule activator. The structures revealed that the S3-S4 linker forms a hydrophobic constriction at the extracellular opening of the pore. Apamin binds to this extracellular constriction and blocks the exit of potassium ions. Furthermore, we identified a structurally related SK2 inhibitor and activator that bind to the transmembrane domains. The compounds exert opposing effects on gating by differentially modulating the conformation of the S6 helices. These results provide important mechanistic insights to facilitate the development of targeted SK2 channel therapeutics.