A benchtop bioreactor to impart coarctation-induced deformation and assess mechanisms of endothelial dysfunction
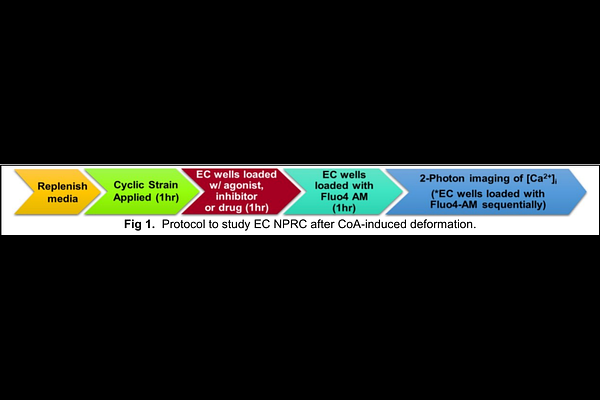
A benchtop bioreactor to impart coarctation-induced deformation and assess mechanisms of endothelial dysfunction
Schock, D. J.; Armstrong, A.; Alli, A. A.; LaDisa, J. F.
AbstractHypertension (HTN) is common with coarctation of the aorta (CoA), a common congenital cardiovascular defect. Coarctation-induced mechanical stimuli can drive remodeling in the proximal aorta, resulting in stiffening and HTN. Natriuretic peptide receptor C (NPRC) expression is downregulated in proximal aortas of hypertensive CoA patients, and preclinical models show endothelial NPRC decreases with increasing coarctation severity. The function of NPRC is regulated by actin cytoskeleton organization. AHNAK potentiates arachidonic acid calcium mobilization in this process, but its role in CoA is unknown. Mechanistic study of NPRC in CoA is hampered by limited, heterogenous patients and lengthy, expensive preclinical models. We developed an in vitro system using human aortic endothelial cells (HAECs) to mimic NPRC and endothelial dysfunction results from CoA and study mechanisms (e.g. AHNAK) regulating NPRC membrane expression. Physiologic (12%) and pathologic (17%) strain conditions derived from preclinical control and CoA aortic measurements were applied to HAECs using a tension bioreactor. Two-photon imaging of the strain-conditioned HAECs response to CNP (agonist) revealed those exposed to pathologic strain had significantly less intracellular calcium [Ca2+]i mobilization than with physiologic strain, consistent with results from intact CoA tissue. Protein expression of NPRC and AHNAK were disrupted in actin cytoskeleton fractions from sucrose density gradient ultracentrifugation studies using lysates of HAEC exposed to pathologic conditions. These results indicate our HAECs straining system mimics the in vivo effects CoA-induced mechanical stimuli have on aortic tissue and can be used can used to conduct studies confirming and/or extending associated mechanisms.