Optogenetic control of Protein Kinase C-epsilon activity reveals its intrinsic signaling properties with spatiotemporal resolution
Optogenetic control of Protein Kinase C-epsilon activity reveals its intrinsic signaling properties with spatiotemporal resolution
Ong, Q.; Lim, C. J. Y.; Liao, Y.; Ng, J. T.-Y.; Lim, L. T. R.; Koh, S. X. Y.; Chan, S. E.; Lee, P. Y. Y.; Lim, H.; Ye, C. R.; Wang, L. C.; Ler, S. G.; Sobota, R. M.; Tan, Y. S.; I. Shulman, G.; Yang, X.; Han, W.
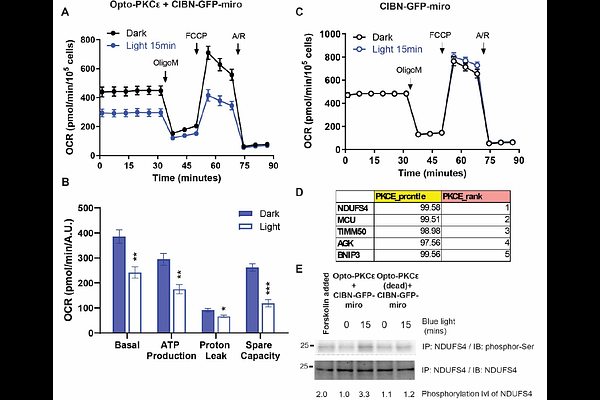
AbstractThe regulation of PKC epsilon (PKCepsilon) and its downstream effects is still not fully understood, making it challenging to develop targeted therapies or interventions. A more precise tool that enables spatiotemporal control of PKCepsilon activity is thus required. Here, we describe a photo-activatable optogenetic PKCepsilon probe (Opto-PKCepsilon) consisting of an engineered PKCepsilon catalytic domain and a blue-light inducible dimerization domain. Molecular dynamics and AlphaFold simulations enable rationalization of the dark-light activity of the optogenetic probe. We first characterize the binding partners of Opto-PKCepsilon, which are similar to those of PKCepsilon. Subsequent validation of the Opto-PKCepsilon tool is performed with phosphoproteome analysis, which reveals that only PKCepsilon substrates are phosphorylated upon light activation. Opto-PKCepsilon could be engineered for recruitment to specific subcellular locations. Activation of Opto-PKCepsilon in isolated hepatocytes reveals its sustained activation at the plasma membrane is required for its phosphorylation of the insulin receptor at Thr1160. In addition, Opto-PKCepsilon recruitment to the mitochondria results in its lowering of the spare respiratory capacity through phosphorylation of complex I NDUFS4. These results demonstrate that Opto-PKCepsilon may have broad applications for the studies of PKCepsilon signaling with high specificity and spatiotemporal resolution.