Structures of the Staphylococcus aureus ribosome inhibited by fusidic acid and fusidic acid cyclopentane
Structures of the Staphylococcus aureus ribosome inhibited by fusidic acid and fusidic acid cyclopentane
Gonzalez Lopez, A.; Larsson, D. S. D.; KIran Koripella, R.; Cain, B. N.; Garcia Chavez, M.; Hergenrother, P. J.; SANYAL, S.; Selmer, M.
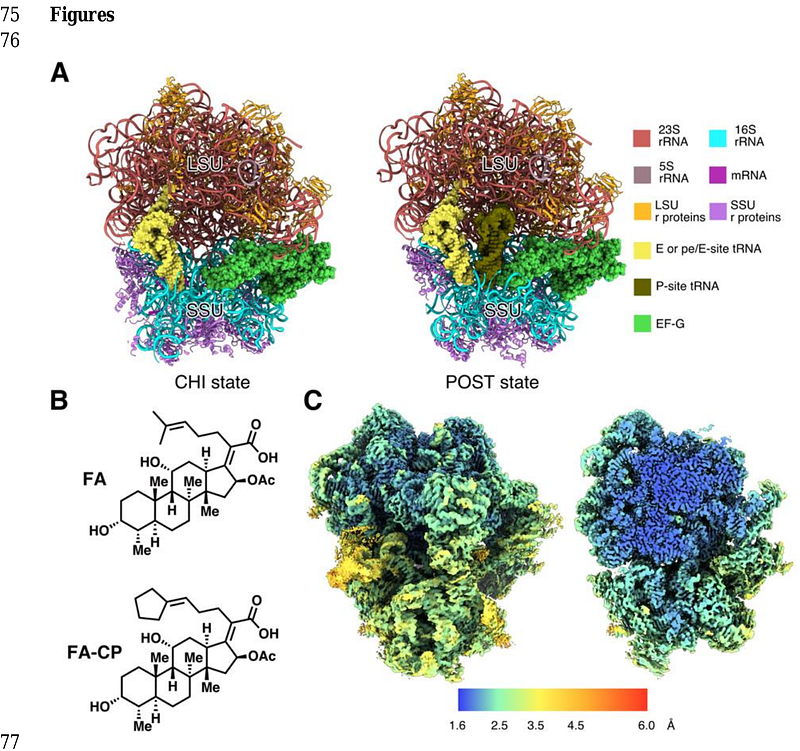
AbstractThe antibiotic fusidic acid (FA) is used to treat Staphylococcus aureus infections. It inhibits protein synthesis by binding to elongation factor G (EF-G) and preventing its release from the ribosome after translocation. While FA is only effective against gram-positive bacteria, the available structures of FA-inhibited complexes are from gram-negative model organisms. To fill this knowledge gap, we solved cryo-EM structures of the S. aureus ribosome in complex with mRNA, tRNA, EF-G and FA to 2.5 [A] resolution and the corresponding complex structures with the recently developed FA derivative FA-cyclopentane (FA-CP) to 2.0 [A] resolution. With both FA variants, the majority of the ribosomal particles are observed in chimeric hybrid state and only a minor population in post-translocational state. As expected, FA binds in a pocket between domains I, II and III of EF-G and the sarcin-ricin loop of 23S rRNA. FA-CP binds in an identical position, but its cyclopentane moiety provides additional contacts to EF-G and 23S rRNA, suggesting that its improved resistance profile towards mutations in EF-G is due to higher-affinity binding. These high-resolution structures reveal new details about the S. aureus ribosome, including confirmation of many rRNA modifications, and provide an optimal starting point for future structure-based drug discovery on an important clinical drug target.