Human tumor suppressor protein Pdcd4 binds at the mRNA entry channel in 40S small ribosomal subunit
Human tumor suppressor protein Pdcd4 binds at the mRNA entry channel in 40S small ribosomal subunit
Brito Querido, J.; Sokabe, M.; Diaz-Lopez, I.; Gordiyenko, Y.; Zuber, P.; Du, Y.; Albacete-Albacete, L.; Ramakrishnan, V.; Fraser, C. S.
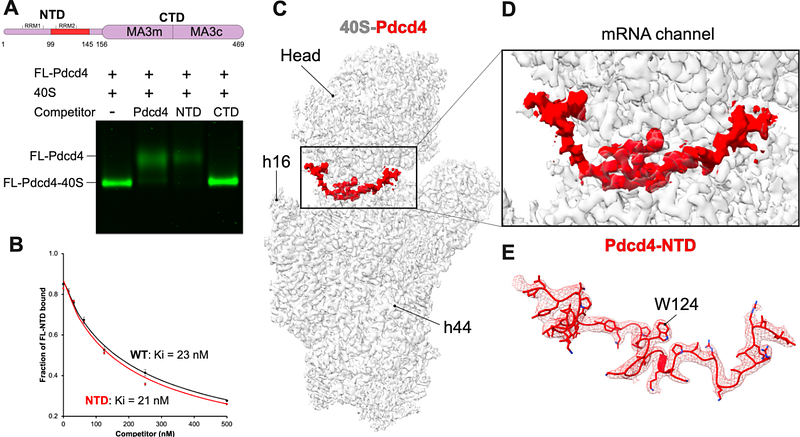
AbstractTranslation is regulated mainly in the initiation step, and its dysregulation is implicated in many human diseases. Several proteins have been found to regulate translational initiation, including Pdcd4 (programmed cell death gene 4). Pdcd4 is a tumor suppressor protein that prevents cell growth, invasion, and metastasis. It is downregulated in most tumor cells, while global translation in the cell is upregulated. To understand the mechanisms underlying translational control by Pdcd4, we used single-particle cryo-electron microscopy to determine the structure of human Pdcd4 bound to 40S small ribosomal subunit, including Pdcd4-40S and Pdcd4-40S-eIF4A-eIF3-eIF1 complexes. The structures reveal the binding site of Pdcd4 at the mRNA entry site in the 40S, where the C-terminal domain (CTD) interacts with eIF4A at the mRNA entry site, while the N-terminal domain (NTD) is inserted into the mRNA channel and decoding site. The structures, together with quantitative binding and in vitro translation assays, shed light on the critical role of the NTD for the recruitment of Pdcd4 to the ribosomal complex and suggest a model whereby Pdcd4 blocks the eIF4F-independent role of eIF4A during recruitment and scanning of the 5\' UTR of mRNA.
