Epithelial-mesenchymal cell state heterogeneity predetermines differential phospho-signaling responses to epidermal growth factor stimulation
Epithelial-mesenchymal cell state heterogeneity predetermines differential phospho-signaling responses to epidermal growth factor stimulation
Kohane, F. V.; Johnstone, C.; Neumann, D. P.; Gunawan, I.; Huang, T.; Vafaee, F.; Chaffer, C. L.; Lock, J. G.
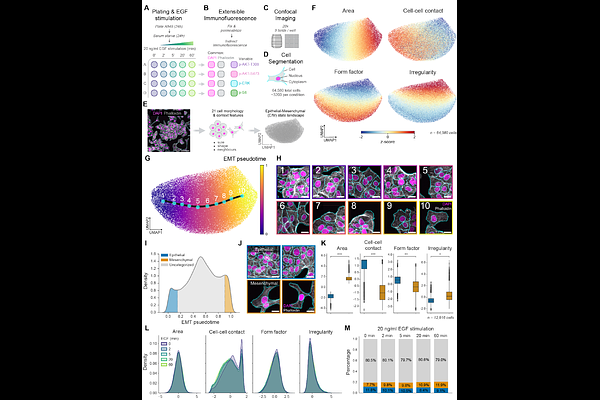
AbstractCancer cell populations appear to show \'noisy\' oncogenic signaling responses to growth factor stimulation, yet how much of this variation is predetermined by prior cell state heterogeneity remains unclear. We here investigate whether epithelial-mesenchymal (E/M) state, a major axis of heterogeneity in cancer cells, influences signaling response to epidermal growth factor (EGF) in A549 non-small cell lung cancer (NSCLC) cells; a signaling axis and disease combination of high clinical significance. We perform confocal imaging of cells immunofluorescently labeled with \'common markers\' (DNA, F-actin) and \'variable markers\' of phospho-signaling (p-AKT-S473, p-AKT-T308, p-ERK1, p-S6) at short intervals (2, 5, 20, 60 minutes) post-EGF stimulation. Single-cell quantitation of cell spatial context-, morphology-, marker level-, and subcellular localization-features was performed on >64,000 cells, fueling representation learning to map all cells within a common, condition-invariant \'E/M state landscape\'. Comparison of E/M state-dependent variations in EGF-stimulated phospho-signaling revealed consistent differences: epithelial cells exhibited rapid, transient activation, while mesenchymal cells showed delayed and sustained responses, particularly in the PI3K/AKT pathway. Subcellular localization of p-AKT also varied dramatically, with epithelial cells establishing early membrane and punctate localizations, while mesenchymal cells maintained sustained nuclear and ruffle-associated AKT activity. Using regression-based \'computational multiplexing\' to infer all phospho-signaling feature values concurrently in all cells, we defined a multi-molecular \'signaling landscape\' and compared signaling response trajectories between epithelial and mesenchymal cells, confirming robust differences in multi-molecular signaling. EGF-induced signaling heterogeneity is thus substantially predetermined by pre-existing E/M states, which dictate distinct intracellular signal localization and signal propagation trajectories. These findings suggest that understanding how heterogeneous E/M states within tumors shape distinctive oncogenic signaling responses will be vital to understanding differential sensitivity to anti-oncogenic signal therapies.