Pharmacodynamic modeling of colistin and imipenem against in vitro Pseudomonas aeruginosa biofilms
Pharmacodynamic modeling of colistin and imipenem against in vitro Pseudomonas aeruginosa biofilms
Guo, Y.; Yin, J.; Aulin, L. B. S.; Ciofu, O.; Moser, C.; Upadhyay, P. J.; Hoiby, N.; Wang, H.; Guo, T.; van Hasselt, J. G. C.
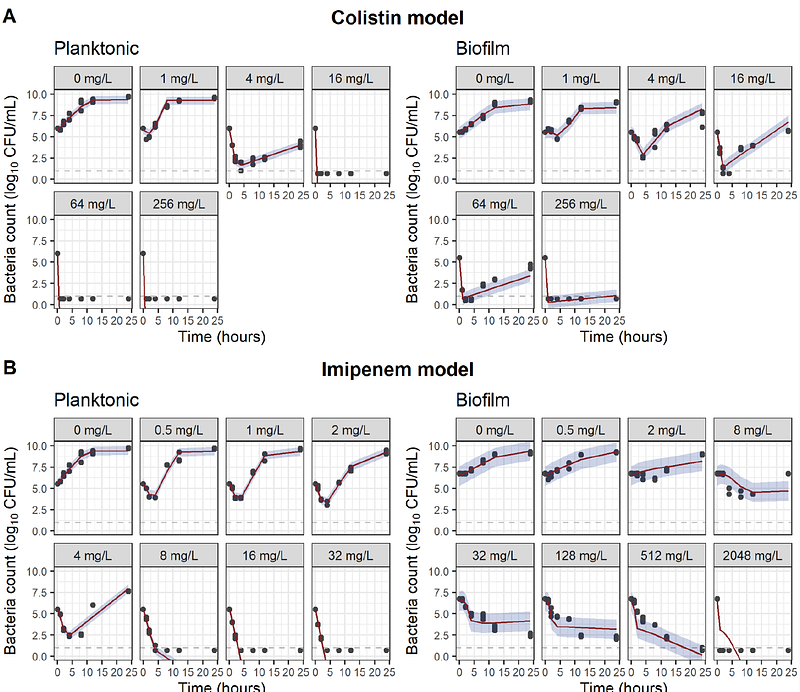
AbstractIntroduction: Antibiotic treatment of chronic biofilm-associated infections can be challenging. Characterization of pharmacokinetic-pharmacodynamic (PK-PD) relationships for biofilm-associated infections may be relevant to inform the design of antibiotic treatment regimens for biofilm-associated infections. To this end, we aim to develop a mathematical PK-PD model for planktonic and biofilm bacterial infections and demonstrate how PK-PD simulations can be used to design optimized dosing schedules, using imipenem and colistin as proof-of-concept examples. Methods: Pharmacodynamic models were developed using time-kill assay data from planktonic and alginate-bead biofilm cultures of Pseudomonas aeruginosa exposed to imipenem or colistin. The PD models were coupled to population PK models for plasma and epithelial lining fluid (ELF) to translate PD relationships for clinical dosing schedules and PK-PD indices. Results: The developed models incorporated sensitive and resistant bacterial subpopulations and were able to adequately capture the observed time-kill data. Simulation studies identified differences in suppression of bacterial growth dynamics for multiple clinical intravenous and inhalation-based treatment regimens and were used to infer biofilm-specific PK-PD indices associated with ELF target site concentrations. Conclusion: In conclusion, we demonstrate the utility of mathematical modeling for the characterization of PK-PD relationships underlying time-kill kinetic profiles in biofilm-associated infections and their utility in translating experimental findings to inform the optimization of clinical dosing schedules.


