Cryo-electron microscopy structures of a high-affinity zinc ABC transporter
Cryo-electron microscopy structures of a high-affinity zinc ABC transporter
Pang, C.; Nguygen, H.; Zhang, Q.; Bahar, I.; Liu, Q.
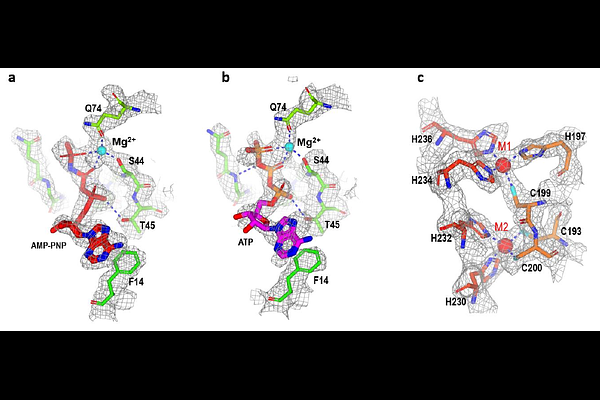
AbstractHow do bacteria acquire scarce zinc; to fuel vital cellular functions? Microorganisms employ high-affinity zinc ABC transporters, yet their structures and regulation are poorly understood. Here, we report cryo-EM structures of the Escherichia coli ZnuB-ZnuC complex, revealing a ZnuB homodimer in an outward-facing, sealed conformation with a central hydrophilic cavity and ZnuC subunits composed of an N-terminal ATP-binding cassette and a C-terminal zinc-sensing domain (ZSD). Zinc binding to the ZSD locks the transporter in an inhibited state regardless of nucleotide, whereas under low-zinc; conditions, ZSD C-terminal disorder permits ATP-driven zinc uptake. These findings clarify the molecular basis of zinc; acquisition and highlight new targets for disrupting metal homeostasis in pathogens.