Thiazole substitution of a labile amide bond - a new option towards stable pantothenamide-mimics
Thiazole substitution of a labile amide bond - a new option towards stable pantothenamide-mimics
Liu, X.; Chu, A.; Nekouei, M.; Lan, C. B.; Auclair, K.; Saliba, K. J.
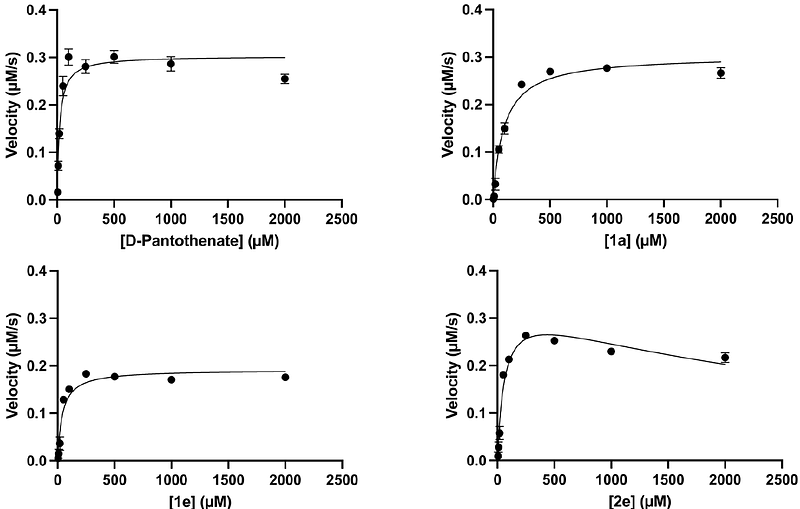
AbstractThe emergence and spread of artemisinin-resistant, malaria-causing P. falciparum provide the impetus for the development of novel antimalarials. Pantothenamides are potent inhibitors of malaria parasite proliferation, however their clinical use is hindered by pantetheinase-mediated degradation in human serum. Here we report the synthesis and biological activity of a series of pantothenamide-mimics in which the labile amide bond is replaced by a thiazole ring with various orientations. Out of 23 novel compounds generated and tested in the presence of pantetheinase, several display sub-micromolar antiplasmodial activity in vitro. A selection of compounds was studied in more detail and CoA biosynthesis and/or utilisation pathways were confirmed to be the target. Toxicity to human cells was not observed. Kinetic studies identified the selected compounds as substrates of the HsPanK3 enzyme, but with much lower affinity compared to that of the natural substrate pantothenate. The most potent thiazole-bearing antiplasmodial compound was found to bind to PfPanK with a 120-fold higher affinity compared to HsPanK, highlighting excellent selectivity, not only against the key first enzyme in the CoA biosynthesis pathway, but also at the whole-cell level. In conclusion, thiazole substitution of the labile amide bond represents a promising avenue for the development of antimalarial pantothenamide-mimics.