Protein tyrosine phosphatase receptor kappa regulates glycolysis and de novo lipogenesis to promote hepatocyte metabolic reprogramming in obesity
Protein tyrosine phosphatase receptor kappa regulates glycolysis and de novo lipogenesis to promote hepatocyte metabolic reprogramming in obesity
Gilglioni, E. H.; Li, A.; Saint-Pierre Wijckmans, W.; Shen, T.-K.; Perez-Chavez, I.; Hovhannisyan, G.; Lisjak, M.; Negueruela, J.; Vandenbempt, V.; Bauza-Martinez, J.; Herranz, J. M.; Ezerina, D.; Demine, S.; Feng, Z.; Vignane, T.; Otero-Sanchez, L.; Lambertucci, F.; Prasnicka, A.; Deviere, J.; Hay, D. C.; Encinar, J. A. N.; Singh, S. P.; Messens, J.; Filipovic, M. R.; Sharpe, H. J.; Trepo, E.; Wu, W.; Gurzov, E. N.
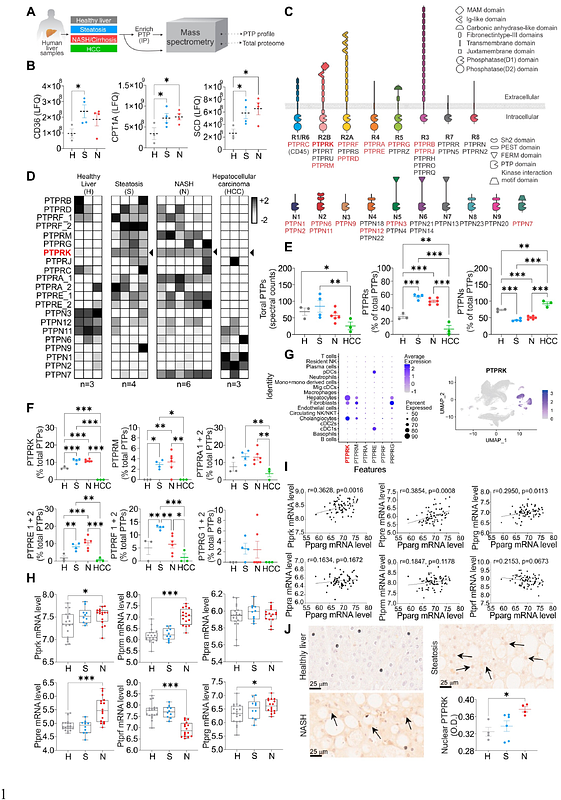
AbstractFat accumulation, de novo lipogenesis, and glycolysis are key drivers of hepatocyte reprogramming and the consequent metabolic dysfunction-associated steatotic liver disease (MASLD). Here we report that obesity leads to dysregulated expression of hepatic protein-tyrosine phosphatases (PTPs). PTPRK was found to be increased in steatotic hepatocytes in both humans and mice, and positively correlated with PPAR{gamma}-induced lipogenic signalling. High-fat-fed PTPRK knockout mice displayed reduced weight gain and hepatic fat accumulation. Phosphoproteomic analysis in primary hepatocytes and hepatic metabolomics identified fructose-1,6 bisphosphatase-1 and glycolysis as PTPRK targets in metabolic reprogramming. Silencing PTPRK in hepatoma cell lines resulted in reduced colony-forming ability, and PTPRK knockout mice developed smaller tumours after diethylnitrosamine-induced hepatocarcinogenesis. Our study defines a novel role for PTPRK in regulating hepatic glycolysis, lipid metabolism, and tumour development. PTPRK inhibition may provide therapeutic possibilities in obesity-associated liver diseases.