Structural basis of multitasking by the apicoplast DNA polymerase from Plasmodium falciparum
Structural basis of multitasking by the apicoplast DNA polymerase from Plasmodium falciparum
Kumari, A.; Enache, T.; Craggs, T. D.; Pata, J. D.; Lahiri, I.
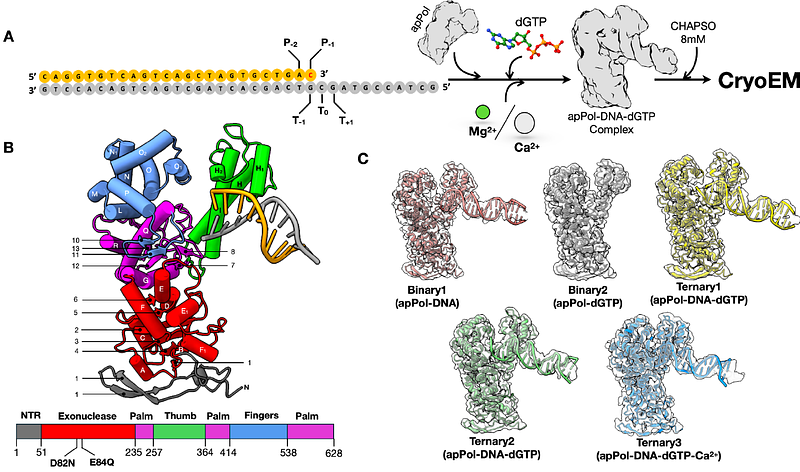
AbstractPlasmodium falciparum is a unicellular eukaryotic pathogen responsible for the majority of malaria-related fatalities. Plasmodium belongs to the phylum Apicomplexa and like most members of this phylum, contains a non-photosynthetic plastid called the apicoplast. The apicoplast has its own genome, which is replicated by a dedicated apicoplast replisome. Unlike other cellular replisomes, the apicoplast replisome uses a single DNA polymerase (apPol) for copying the apicoplast DNA. Being the only DNA polymerase in the apicoplast, apPol is expected to multitask, catalysing both replicative and lesion bypass synthesis. Replicative synthesis typically relies on a restrictive active site for high accuracy while lesion bypass requires an open active site. This raises the question how does apPol combine the structural features of multiple DNA polymerases in a single protein. Using single particle electron cryomicroscopy (cryoEM), we have solved the structures of apPol bound to its DNA and nucleotide substrates in five pre-chemistry conformational states, allowing us to describe the events leading up to nucleotide incorporation and answer how apPol incorporates features of multiple polymerases. We found that, unlike most replicative polymerases, apPol can accommodate a nascent base pair with the fingers in an open configuration, which might facilitate the lesion bypass activity. In the fingers open state we identified a nascent base pair checkpoint that can preferentially select a Watson-Crick base pair, an essential requirement for replicative synthesis. Taken together these structural features explain how apPol may balance replicative and lesion bypass synthesis.