The action mechanism of actinoporins revealed through the structure of pore-forming intermediates
The action mechanism of actinoporins revealed through the structure of pore-forming intermediates
Arranz, R.; Santiago, C.; Masiulis, S.; Rivera-de-Torre, E.; Palacios-Ortega, J.; Carlero, D.; Heras-Marquez, D.; Gavilanes, J. G.; Arias-Palomo, E.; Martinez-del-Pozo, A.; Garcia-Linares, S.; Martin-Benito, J.
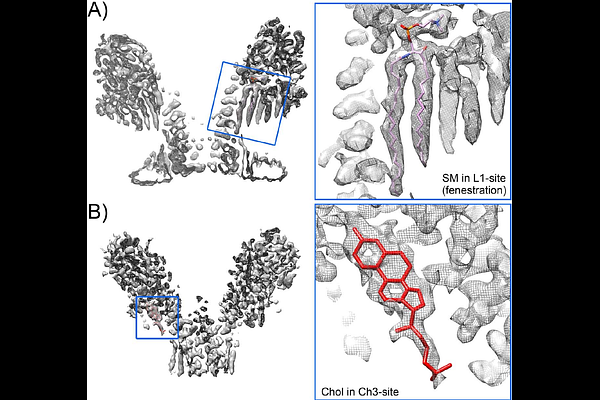
AbstractPore-forming proteins exemplify the transformative potential of biological molecules. Initially produced in a monomeric, water-soluble form, they spontaneously assemble into multimeric integral membrane proteins in the presence of suitable target lipids. Their functions include roles in apoptosis, cell signaling, immunity, as well as attack and defense systems between different organisms. This latter group encompasses actinoporins, a family of pore-forming toxins from sea anemones that kill target cells by perforating their plasma membrane. Here, we have determined the structures of two such toxins, fragaceatoxin C and sticholysin II, in a membrane environment using cryogenic electron microscopy. The structures reveal how dozens of lipid molecules interact in an orderly manner, forming an intrinsic part of the pore. We have also isolated different pore-forming intermediates, where only a fraction of the constituent monomers is incorporated, exhibiting non-closed, arc-shaped structures. Based on these structures we propose a mechanism of action where the sequential assembly of toxin monomers onto the membrane, accompanied by conformational changes, triggers pore formation and membrane perforation. Our results contribute to a better understanding of the transforming capacity of these pore-forming proteins, which are becoming increasingly important for their diverse biotechnological applications.