Phosphatase activity is dispensable for PRL-3-mediated oncogenesis and tumor progression
Phosphatase activity is dispensable for PRL-3-mediated oncogenesis and tumor progression
Jolly, J. T.; Al-Hamaly, M. A.; Smith, C. N.; Spielmann, H. P.; Blackburn, J. S.
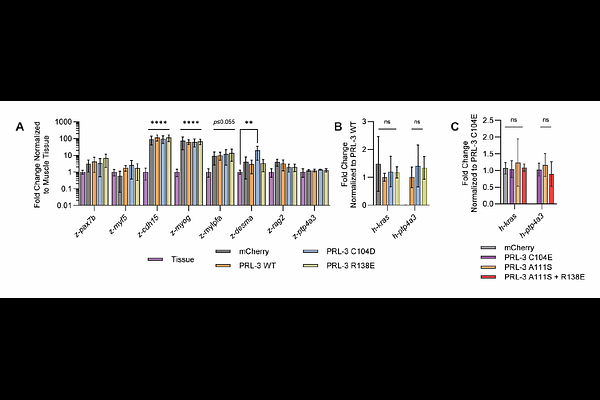
AbstractPhosphatase of Regenerating Liver 3 (PRL-3) is frequently upregulated in cancer and associated with poor prognosis, yet its oncogenic mechanism remains unresolved. Although traditionally studied for its phosphatase activity, PRL-3 also engages in protein-protein interactions via its catalytic site, notably binding the CNNM magnesium transporters, rendering these functions mutually exclusive. The commonly used C104S mutant disrupts both activities, confounding interpretations of prior studies. To dissect the distinct functions of PRL-3, we employed mutants selectively deficient in phosphatase activity (C104D) or CNNM binding (R138E). In zebrafish models of acute lymphoblastic leukemia (ALL) and rhabdomyosarcoma (RMS), as well as in human cancer cell lines, both wild-type PRL-3 and C104D enhanced tumor initiation, growth, and dissemination, while R138E had no effect. These findings indicate that PRL-3 promotes cancer via non-catalytic mechanisms. To support therapeutic development, we established an in vitro FRET-based assay to screen for inhibitors of the PRL-3:CNNM interaction and validated a cyclic peptide as a positive control. By demonstrating that PRL-3 enhances cancer progression independently of its catalytic activity, this study shifts focus toward targeting its binding functions as a therapeutic strategy.