An intricate balancing act: Upstream and downstream frameshift co-regulatory elements
An intricate balancing act: Upstream and downstream frameshift co-regulatory elements
Lee, S.; Yan, S.; Dey, A.; Laederach, A.; Schlick, T.
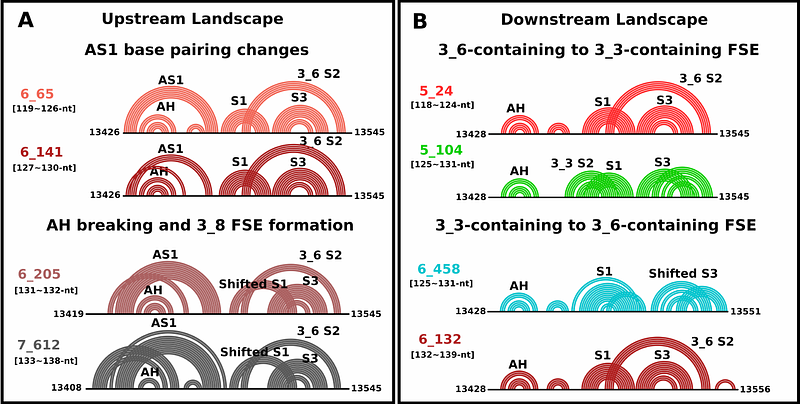
AbstractTargeting ribosomal frameshifting has emerged as a potential therapeutic intervention strategy against Covid-19. During ribosomal translation, a fraction of elongating ribosomes slips by one base in the 5\' direction and enters a new reading frame for viral protein synthesis. Any interference with this process profoundly affects viral replication and propagation. For Covid-19, two RNA sites associated with ribosomal frameshifting for SARS-CoV-2 are positioned on the 5\' and 3\' of the frameshifting residues. Although much attention has been on the 3\' frameshift element (FSE), the 5\' stem-loop (attenuator hairpin, AH) can play a role. The formation of AH has been suggested to occur as refolding of the 3\' RNA structure is triggered by ribosomal unwinding. However, the attenuation activity and the relationship between the two regions are unknown. To gain more insight into these two related viral RNAs and to further enrich our understanding of ribosomal frameshifting for SARS-CoV-2, we explore the RNA folding of both 5\' and 3\' regions associated with frameshifting. Using our graph-theory-based modeling tools to represent RNA secondary structures, \"RAG\" (RNA-As-Graphs), and conformational landscapes to analyze length-dependent conformational distributions, we show that AH coexists with the 3-stem pseudoknot of the 3\' FSE (graph 3_6 in our dual graph notation) and alternative pseudoknot (graph 3_3) but less likely with other 3\' FSE alternative folds (such as 3-way junction 3_5). This is because an alternative length-dependent Stem 1 (AS1) can disrupt the FSE pseudoknots and trigger other folds. In addition, we design four mutants for long lengths that stabilize or disrupt AH, AS1 or FSE pseudoknot to illustrate the deduced AH/AS1 roles and favor the 3_5, 3_6 or stem-loop. These mutants further show how a strengthened pseudoknot can result from a weakened AS1, while a dominant stem-loop occurs with a strengthened AS1. These structural and mutational insights into both ends of the FSE in SARS-CoV-2 advance our understanding of the SARS-CoV-2 frameshifting mechanism by suggesting a sequence of length-dependent folds, which in turn define potential therapeutic intervention techniques involving both elements. Our work also highlights the complexity of viral landscapes with length-dependent folds, and challenges in analyzing these multiple conformations.


