Bright Cells, and Inefficient Enzymatic Activity in a Renin Hypomorph Mouse
Bright Cells, and Inefficient Enzymatic Activity in a Renin Hypomorph Mouse
Medrano, S.; Almeida, L.; Ruiz-Perez, F.; Gutierrez-Hernandez, A.; Yamaguchi, H.; Matsuoka, D. M.; Yamaguchi, M.; Smith, J. P.; Wagamon, T.; Sequeira-Lopez, M.; Gomez, R. A.
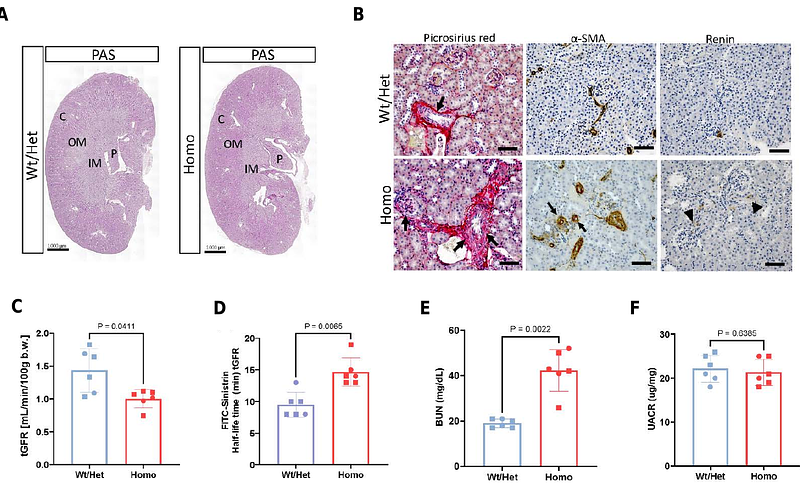
AbstractJuxtaglomerular (JG) cells are crucial regulators of blood pressure and fluid-electrolyte homeostasis. Under normal conditions, renin secretion by JG cells is sufficient to maintain homeostasis. However, under physiological stress such as narrowing of one of the renal arteries, heart failure, dehydration, or chronic administration of renin-angiotensin system (RAS) inhibitors, additional cells along the renal arterioles are transformed to the renin phenotype to meet the demands for renin and regain homeostasis. In cases of prolonged and persistent stimulation of renin cells, concentric arteriolar hypertrophy develops. The study of renin cell identity, plasticity and function often requires the isolation of this rare cell type. Here, we report on the generation of a mouse model to label renin-expressing cells with a bright fluorescent reporter under control of the Ren1c locus for the tracking and isolation of renin cells. Kidneys from adult heterozygous (Het) Ren1ctdTomato/+ mice showed tdTomato signal confined to the JG area under basal conditions, and extending along the afferent arterioles and in the intraglomerular mesangium upon treatment with captopril + low-salt diet to induce the endocrine transformation of renin cells. Unexpectedly, homozygous (Homo) Ren1ctdTomato/tdTomato mice exhibited increased tdTomato signal that extended along the afferent arterioles and into the mesangium even under normal physiological conditions, with progressive thickening of the kidney arterioles with age. Despite reduced renin immunostaining in the renal cortex, Ren1ctdTomato/tdTomato Homo mice exhibited significantly higher kidney Ren1 mRNA and circulating renin levels when compared to Het controls. Moreover, Homo mice showed significantly lower blood pressure measured under anesthesia and angiotensin I (Ang I) plasma levels, indicating compromised renin activity. In addition, Homo mice developed interstitial fibrosis and compromised kidney function. The concentric arteriolar hypertrophy phenotype observed in these mice is identical to that described when RAS is genetically or pharmacologically inhibited, including the presence of mutations in the renin gene. Unlike mice with global deletion of renin, these animals did not require neonatal saline injections to survive and did not develop other kidney abnormalities, indicating that the bicistronic approach rendered a renin hypomorphic mouse. Ren1ctdTomato mice constitute an excellent model for the bright and strong labeling of renin-expressing cells and for the study of the mechanisms involved in the development of concentric vascular hypertrophy under RAS inhibition. In addition, this model may provide a better understanding of factors controlling renin protein folding, stability, packaging, and release.