Cell-Based Potency Assay for Anti-CD3-Anti-CD19 Diabody
Cell-Based Potency Assay for Anti-CD3-Anti-CD19 Diabody
Weiss, T. L.; Paniagua, J.; Johnson, T. M.; Cripe, T. P.; Ralph, P.
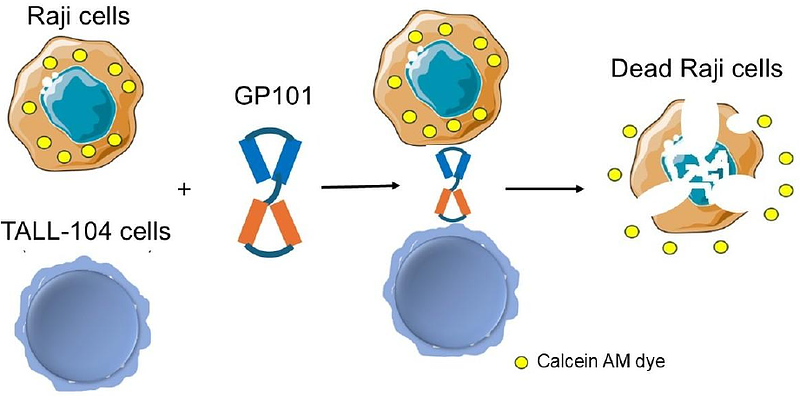
AbstractA cytotoxicity assay was developed to measure the potency of GP101, a single chain diabody. GP101 is comprised of two linked Fv domains, with one that binds CD3 (expressed on T cells), and the other that binds CD19 (expressed on B cells). GP101 directs CD3 positive T lymphocytes to CD19 positive B lymphocytes. This immunotherapy redirects CD3+ T cells to CD19-expressing B-cell malignancies, enabling T-cell-mediated tumor cell lysis. We developed a GMP cell-based potency assay to satisfy the FDA requirements. The potency assay uses a cytotoxic T cell line, and a B cell lymphoma cell line as the target. The target lymphoma B cell line is prelabeled with a fluorescent dye, and upon T cell mediated killing, the fluorescence dye is released and detected using a fluorometer. The emitted fluorescence is proportional to the dose of GP101. Greater than 90% of the target B cells were killed within two hours exposure in vitro with the lowest amount of detectable killing at 60pg/mL GP101. The assay is suitable for measuring purified GP101, or GP101 expressed by cells transduced by GP101 plasmid or AAV preparations, and bioactivity in animal or human blood. This novel assay met GMP/GLP compliance, allowing a quantifiable and reproducible measure of efficacy, ensuring batch-to-batch consistency, and met safety and effectiveness regulatory requirements. This potency assay may be applicable for testing other CD3-CD19 T cell engagers and suitable for developing other diabody mediated potency assays with appropriate antigens.