Microvascular homeostasis is compromised in pancreatic islets in a mouse model of beta cell loss and low-grade inflammation
Microvascular homeostasis is compromised in pancreatic islets in a mouse model of beta cell loss and low-grade inflammation
Mateus Goncalves, L.; Shirvaikar, I.; Sideris, K.; Pereira, E.; Slak Rupnik, M.; Almaca, J.
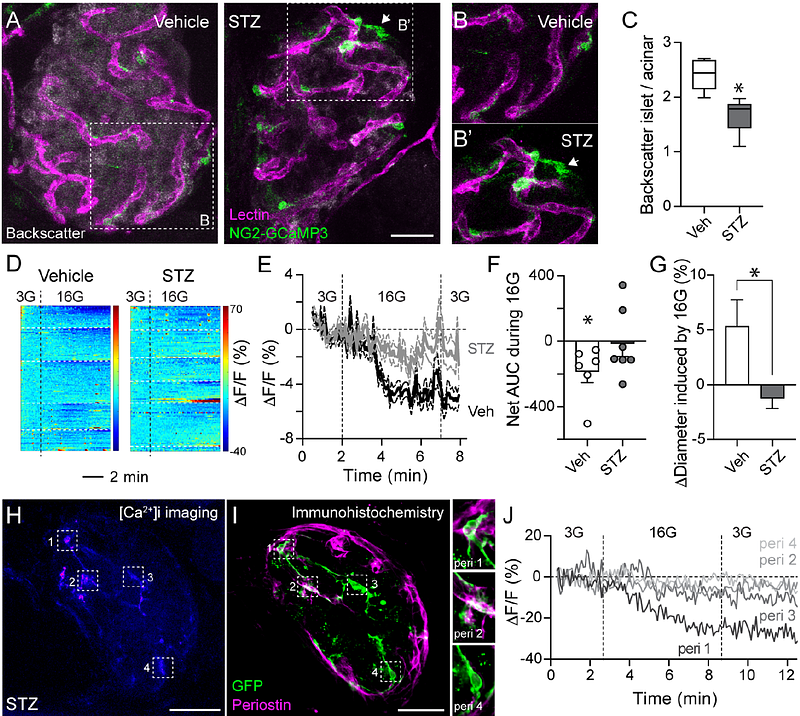
AbstractVascular dysfunction is considered a consequence of diabetes. However, in pancreatic islets, some haemodynamic changes occur before the onset of symptoms. The underlying mechanisms driving islet vascular abnormalities have not been fully characterized, but islet pericyte dysfunction appears to be an early event in the pathogenesis of human type 1 diabetes (T1D). It remains to be investigated, however, how abnormal pericyte physiology affects their ability to regulate islet blood flow and vascular permeability. To address this issue, we treated mice with multiple subdiabetogenic doses of the beta cell toxin streptozotocin (STZ; 50mg/kg) and recorded islet vascular responses when animals developed glucose intolerance but were still not diabetic (average fed glycemia <200 mg/dL). At this stage, accompanying increased macrophage density, islet pericytes adopted a myofibroblast-like appearance and interacted closely with endothelial cells expressing high levels of the adhesion molecule ICAM-1. This phenotypical switch of pericytes in STZ-treated mice had functional repercussions: it impacted glucose-induced vasomotor responses ex vivo in living pancreas slices and hyperglycemia-stimulated increases in islet blood flow recorded in vivo in the exteriorized pancreas. Impaired vasomotor responses were accompanied by enhanced extravazation of a fluorescent dextran (500 kDa) from vessels and accumulation in the interstitial space surrounding islets in STZ-treated mice. Our study indicates that abnormal pericyte function, and compromised capacity to regulate blood flow and vascular integrity, are part of a pathogenic process occurring in islets before diabetes onset, associated with a loss of functional beta cell mass and inflammation.