A perfusion-independent high-throughput method to isolate liver sinusoidal endothelial cells
A perfusion-independent high-throughput method to isolate liver sinusoidal endothelial cells
Babin-Ebell, A.; Mao, Y.; Baljkas, T.; Pilz, F.; Winkler, M.; Kuerschner, S.; Hoffarth, M.; Goerdt, S.; Reiners-Koch, P.-S.; Singhal, M.
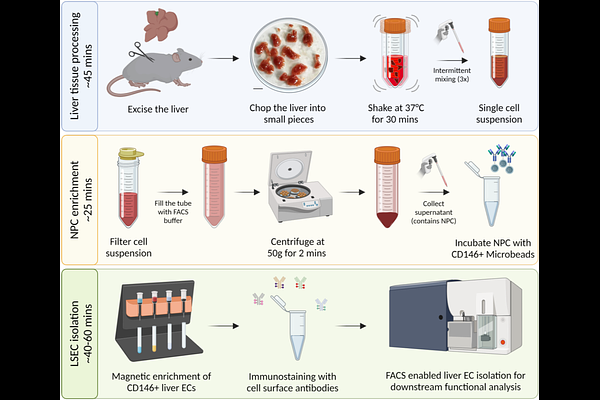
AbstractLiver sinusoidal endothelial cells (LSECs) critically regulate homeostatic liver function and liver pathogenesis. However, the isolation of LSECs remains a major technological bottleneck in studying molecular mechanisms governing LSEC functions. Current techniques to isolate LSECs, relying on perfusion-dependent liver digestion, are cumbersome with limited throughput. We here describe a perfusion-independent high-throughput procedure to isolate LSECs with high purity. Indifferently from previous perfusion-independent approaches, we coarsely chopped liver tissue into 5 mm diameter fragments and incubated them in the digestion mix for 30 minutes with intermittent mixing with a 5 ml pipette. This led to the safeguarding of LSEC integrity and yielded 8.5 +/- 1.0 million LSECs per liver, which is comparable to previously reported yields for perfusion-dependent protocols for isolating LSECs. Combining magnetic and fluorescence-activated cell sorting, LSECs from different zones of the hepatic sinusoid can now be isolated in high numbers in less than 2 hours for downstream applications including proteomics. These technical advancements reduce post-mortem changes in the LSEC state and aid in reliable investigation of LSEC functions.